Scope of metallization of plastics
The scope of application of metallization is the production of finished products from dielectrics, which, having some properties of a metal part, have less weight and are much cheaper to produce. For example, parts made using injection molding machines or parts made by injection molding. Such parts can be used in industries where the mass of parts is important, for example, in the aircraft industry. A separate area is the production of conductive layers in the manufacture of printed circuit boards.
This type of electroplating, known as galvanoplasty, is essentially the process of applying a layer of metal to a mold or workpiece made of wax, plaster or other plastic material. Using electroplating methods, metal copies of art objects, bas-reliefs, sculptures, various artistic products such as metallized tree leaves, flowers, fruits and much more are made.
Ion-vacuum sputtering
The main advantage of this method is that there is no need to heat the evaporator very much. The metal is atomized by bombardment with negatively charged gas ions. The creation of such an environment is possible thanks to special discharges inside the working chamber. For this purpose, the equipment uses a magnetic cooling system. A glow discharge for sputtering the sprayed element is created between 2 electrodes due to the supply of high voltage voltage up to 4 kV. A gas environment with a pressure of up to 0.6 Pascal is created in the working chamber. Vacuum ion-plasma sputtering is also performed using specialized equipment using a similar principle.
Main tasks of metallization
Depending on the area of application of metallized parts, the galvanizer is faced with diametrically opposed tasks. If in the first case, in order to obtain a high-quality finished product from metallized plastic, it is necessary, first of all, to ensure the strength of adhesion of the metal layer to the surface of the dielectric, then when implementing electroforming methods it is necessary to ensure free removal of the mold, often without its destruction.
In electroplating, this problem is solved quite simply; highly dispersed graphite or a special conductive varnish is used to form a thin layer of conductive material for the purpose of further applying a layer of nickel, copper or other metal to the surface of the product by the galvanic method. We will write more about electroplating methods, various techniques, and process features in one of the following articles; now we will focus on the production of finished parts from dielectrics using the metallization method.
As stated above, to obtain a high-quality product, it is first necessary to ensure sufficient adhesion strength of the metal layer to the surface of the dielectric. For plastic products, an adhesion strength of 0.8-1.5 kN/m for peeling and 14 MPa for peeling is considered sufficient. These values are ensured by thoroughly preparing the surface of the part before applying the metal layer, as well as by monitoring the uniformity of the composition of the initial raw materials of the parts. For products produced by injection molding or molding, the use of any release agents, which could have a negative effect on the adhesion strength of the layer and the base, is excluded.
Technological features of metallization
Copper is most often used as an underlayer surface for electroplating. It is the copper layer that will play the role of a damper for the plastic, due to which the stress will be stabilized, which is inevitable with a significant difference in the thermal stress coefficient of such dissimilar materials. the sublayer will be additionally chromed or nickel plated, as described below.
Layer composition:
- Plastic.
- Matte copper layer.
- Copper layer with shine.
- Metal with a chemical type of deposition.
- Nickel shiny layer.
- Semi-shiny nickel finish.
- Nickel matte layer.
- Chrome layer with shine.
- Conversion layer.
- Shiny and matte metallic layer.
The structural components that are applied to the electrically conductive sublayer of the coating can vary greatly. We can talk about films of shiny, velor, bleached, blackened, patinated and other types.
The purpose of films is not simply to improve the appearance of products. For example, nickel plating will extend the life of the plastic. The fact is that nickel can compress plastic, greatly strengthening the material.
The features of the structural composition that will be applied to the electrically conductive coating layer can vary greatly. We will talk about films of bleached, shiny, blackened, velor, patinated and other types. The purpose of films is not only to improve the appearance of products. For example, nickel-plated coatings extend the service life of plastics. The fact is that nickel can inhabit plastic, significantly strengthening the material. To create a galvanic coating, an electrolyte is required.
There are different types of electrolytes used, including:
- Shiny copper plating.
- Electrolytes for nickel plating.
- Specialized compositions on the basis of which velor-type coatings or coatings interspersed with solid particles will be created.
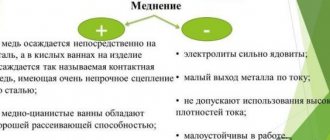
Other metals, such as zinc or tin, should also be used. But before applying these types of metals, passivation will be required, after which a film will appear on the surface (with or without color). These types of films will protect the material from rust or plaque. Chemical metallization of plastics is characterized by the fact that metal-type sublayers do not have high electrical conductivity. In any case, the conductivity will be much lower than in the case of an electrolyte.
For this reason, during electrochemical deposition, the current density used should be small - from 0.5 to 1 Ampere per square decimeter. If the density is higher, a bipolar effect will appear, which will lead to the dissolution of the coating near the place where there is contact with the conductive suspension. In certain cases, to avoid dissolution of the coating, nickel or copper will be applied to the chemically deposited metal layer. In this case, all this is done at a low electric current density, but further layers will be applied in the standard mode.
Metallization methods and surface preparation
There are three methods of metallization - physical, chemical and galvanic, which allow solving various problems and require their own approaches to preparing the surface of dielectrics for the metallization process. A universal method that allows us to obtain products with the highest possible characteristics is the galvanic (electrochemical) method, which is divided into several stages:
- mechanical preparation of the surface of parts - removal from the surface of waste material remaining during manufacturing (molding or casting), cleaning of recessed areas (grooves, holes), etc.;
- chemical surface preparation – degreasing and etching;
- sensitization and activation of the surface with special compounds and reagents;
- applying a conductive sublayer using a chemical method;
- applying galvanic coating to a metallized surface.
The task of the galvanizing department specialists is to ensure that, as a result of these stages, the basic conditions for obtaining a high-quality coating are ensured - the necessary cleanliness of the surface of the part, the specified roughness and the absence of organic substances on the surface.
Mechanical methods of surface preparation depend on the material of the product and the method of manufacturing the initial parts and, as a rule, come down to a simple operation of mechanically cleaning the surface from production waste.
Degreasing of the surface of plastic parts is carried out in a solution containing:
- trisodium phosphate 30-40 g/l;
- sodium hydroxide 8-10 g/l;
- sodium liquid glass 5-7 g/l;
- sodium carbonate 40-45 g/l.
The process takes place at a temperature of 40-500C for 3-5 minutes.
The adhesive properties of a metal coating largely depend on the quality of etching of the parts. During the etching process, micropores and microcracks are formed on the surface, which provide sufficient adhesion strength of the coating to the base. For etching, a solution is used that is almost similar in composition to the chromium plating electrolyte - 100 g/l sulfuric acid and 30 g/l chromic anhydride. The process takes place at a temperature of 600C for 1-5 minutes.
Surfaces suitable for spraying
Any objects that can withstand heating up to 80 °C and exposure to specialized varnishes are suitable for spraying. The advantage of the technology is that to give products the effect of copper coatings, mirror chrome plating, gold plating, nickel plating, there is no need to pre-polish the surfaces. More often, vacuum metallization is used to coat parts made of plastic, glass, metal alloys, and various polymer and ceramic products. Less frequently, but still, the technology is used for softer materials such as wood, textiles, and fur.
Plastic metallization process
Sensitization is the process of chemically depositing a thin layer of catalyst on the surface. Plastic products are placed in a solution of 30-40 g/l tin dichloride and 30-40 g/l hydrochloric acid at shop temperature, then washed in distilled water and the surface is activated in a solution of 1-2 g/l palladium dichloride and 1-2 ml/l hydrochloric acid for 3-5 minutes. The thin layer of palladium that forms as a result catalyzes the deposition of copper from the electroless copper plating solution:
- copper sulfate 100 g/l;
- caustic soda 100 g/l;
- sodium carbonate (anhydrous) 30 g/l;
- glycerin 100 g/l;
- formalin 33% 25-35 ml/l.
The chemical deposition process of copper takes place at shop temperature for 20 minutes. As a result, a ready-made metal sublayer is obtained for further galvanic deposition of metal. The galvanic process takes place in standard electrolytes of nickel plating, copper plating, tin plating or chrome plating and differs from classical electroplating only in the features of fastening conductive contacts. The metallization process is also completed in the standard way - the parts are washed and dried.
You may be interested in the following articles:
|
Nickel plating of plastics
After activation (without rinsing!) the parts are immediately transferred to a nickel plating . There is a choice of solutions (g/l):
- Nickel sulfate - 30, sodium hypophosphite - 10, sodium acetate - 10. Solution temperature 90 °C, metal film growth rate 15 µm/h.
- Nickel chloride - 30, sodium hypophosphite - 10, sodium citrate - 100, ammonium chloride to pH = 8-9. Solution temperature 90 °C, growth rate 6 µm/h.
- Nickel chloride - 30, sodium hypophosphite - 10, sodium citrate - 10. Solution temperature 85 °C, growth rate 5 µm/h.
Solutions are prepared in the following sequence. First, all components except sodium hypophosphite are dissolved in most of the water. It is separately dissolved in a small part of water. Immediately before loading the parts, both solutions are mixed.
Equipment for vacuum deposition
Vacuum metallization plants are quite complex and expensive equipment that consume a lot of electricity. To create a complex technological cycle, a fairly spacious room is required, since several multifunctional devices must be placed. Main components of the vacuum system:
- Power supply and control unit in conjunction with a source of condensed metals.
- Gas distribution system that creates a vacuum space and regulates gas flows.
- Working chamber for vacuum metallization.
- Unit for thermal control, control of spraying thickness and speed, coating properties.
- A transporting unit responsible for changing the position of workpieces, feeding them and removing them from the chamber.
- Unit blocking devices, gas filters, dampers and other auxiliary equipment.
Magnetron and ion-plasma vacuum equipment comes in different sizes, from small, with chambers of several liters, to very large, with chamber volumes of several cubic meters.
has sufficient production capacity and appropriate equipment to provide various methods of vacuum deposition. From us you can order ion-plasma coating of products made from any materials with metals such as titanium, copper, aluminum, brass, chrome, various alloys, etc. We guarantee high quality work and reasonable prices.
For clients in Moscow
We will be happy to answer any questions by calling our Moscow office:
So friends, everything worked out! As planned)) While the most stubborn continue to eat the cactus and restore the plastic ones!
o_O Visteon2, a group of enterprising comrades =)), having cooperated and found each other)) did it - namely, they restored the metal reflectors of the Visteon1 lenses (which were installed at one time on the pre-Restyle Mondeo4, Land Rover, Jaguar, Volvo and a bunch of other brands) by vacuum deposition of aluminum. It was done in the St. Petersburg JSC "VIMA" (not an advertisement! For the sake of truth) ... for the clear, without a hitch, organization of this event, I express categorical respect and respect! 111 Denis (aka dionis-spb) Denis, you are a man! good!)) thank you so much again, nothing would have happened without you!
There's only a little left! put it in place... as the men used to say at my first job: “... - start and finish. "=))
stay tuned! it will be interesting)
PS The budget for this action is total! taking into account the purchase of used modules, their triple transportation (to me, from me to St. Petersburg and ready back to me)... specifically, the restoration itself cost about 1,700 rubles. for a pair of reflectors, according to the small wholesale price list =)) from the previous entry
Issue price: 3,700 ₽