Non-alloy steel alloy is the chemical term used to name two types of steel. Steel is a metal alloy. It is made up of iron and some other elements such as carbon. Unalloyed steels do not have elements added to the steel when it is remelted. Steel is widely used all over the world due to several reasons such as low cost, ease of manufacture, strength, etc. There are different grades of steel available according to their properties.
Alloy steel
is a type of steel and has a large number of other elements besides iron and carbon. The main difference between alloy and unalloyed steel is that in alloy steel, the remaining elements are added to iron during smelting whereas in unalloyed steel, no elements are added during smelting.
Read also: What to do if bitten by a midge
There are two main types of alloys as substitution alloys and interstitial alloys. When molten metal is used in the production of alloys, the sizes of the atoms will determine what type will be formed. If the metal atoms that come together to be mixed have relatively equal sizes, a substitute type of alloy is formed, but if one type of metal atom is smaller than another type, an intermediate alloy is formed.
Main Difference – Alloy vs Unalloy Steel
Alloy and non-alloy steel are the chemical terms used to refer to the two types of steel. Steel is a metal alloy. It is made up of iron and some other elements such as carbon. Unalloyed steel has no elements added to the steel during smelting. Steel is widely used all over the world for several reasons such as low cost, ease of production, strength, etc. There are different grades of steel depending on their properties. Alloy steel is a type of steel and has a large number of other elements besides iron and carbon. The main difference between alloy and unalloy steel is that in alloy steel, other elements are added to the iron during smelting, whereas in unalloy steel, no elements are added during smelting.
Key areas covered
1. What is alloy steel - definition, properties 2. What is unalloy steel - definition, properties 3. What is the difference between alloy and unalloy steel - Comparison of the main differences
Key Terms: Alloy, Alloy Steel, Carbon, Chromium, High Alloy Steel, Intermediate Alloy, Iron, Metal, Unalloy Steel, Low Alloy Steel, Melt, Steel, Substitute Alloy
Classification and brands
There are several main criteria by which carbon grades are divided. One of the most important among them is the conditions for deoxidation. The following low-carbon steels are distinguished:
- Calm. Includes a minimal content of iron oxide, which makes the smelting process “calm” - without the violent release of carbon dioxide from the metal surface. This became possible thanks to the introduction of deoxidizing agents: aluminum, manganese and silicon. All escaping gases accumulate in the shrinkage cavity, which is subsequently cut off, resulting in a dense and homogeneous metal.
- Boiling. They are deoxidized by manganese alone. They have an increased amount of iron oxide in their composition. The smelting process is accompanied by the release of carbon dioxide, which gives the impression that the metal is boiling. These steels are less durable and less homogeneous in chemical composition, but at the same time they are cheap and have a low percentage of waste in production.
- Semi-calm. In addition to manganese, aluminum is also used to remove oxygen. According to the characteristics, this carbon steel is something between boiling and calm alloys.
In addition to the degree of deoxidation, low-carbon grades are also classified according to the presence of non-metallic inclusions in their composition. Based on this, they differ in:
Difference between low alloy steel and high alloy steel
The main difference between low alloy steel and high alloy steel is that low alloy steels contain less than 0.25% alloying element, whereas high alloy steels have more than 10% alloying element .
In addition to dividing into low-alloy and high-alloy steel, it is also divided according to the degree of alloying into medium-alloy. In this steel, the amount of alloying elements ranges from 2.5 to 10%)
An alloy is a mixture of two or more elements. It is produced by mixing a metal with some other elements (metals or non-metals or both) to produce a material that has improved properties over the original metal. Low alloy steel and high alloy steel are two types of iron alloys with alloying elements.
The most popular alloying elements in these steels are: nickel (Ni), copper (Cu), titanium (Ti) and vanadium (V), nitrogen (N), etc.
Content
- Overview and main differences
- What is low alloy steel
- What is high alloy steel
- What is the difference between low alloy steel and high alloy steel
- Conclusion
What is low alloy steel?
Low alloy steel is a type of alloy steel whose properties are improved compared to carbon steel. For example, this alloy has better mechanical properties and greater corrosion resistance than carbon steel. The carbon content of low alloy steel is less than 0.2%. The most common alloying elements in this steel are: Nickel (Ni), Chromium (Cr), Molybdenum (Mo), Tungsten (V), Boron (B), Tungsten (W) and Copper (Cu).
Sheet steel
In most cases, the manufacturing process of these alloy steels includes heat treatment and tempering (for normalization). But now, there is a tendency to harden and temper. In addition, almost all low alloy steel materials are weldable. However, the material sometimes requires treatment before or after welding (to avoid cracking).
Some advantages of low alloy steel:
- Yield strength is higher
- High tensile strength
- Higher resistance to oxidation and corrosion
- Low cold brittleness threshold
This material is used in industry, but up to a maximum temperature of 580 °C. If the temperature is higher than 580 °C, this material is not suitable due to lack of sufficient oxidation resistance to cope with high temperatures.
What is high alloy steel?
High alloy steel is a type of alloy steel that contains more than 10% alloying elements. Unlike low alloy steel, the alloying elements for high alloy steel are chromium (Cr) and nickel (Ni). The most famous example of this steel is stainless steel.
Stainless steel pan
Chromium provides steel with a thin oxide layer on the surface of the steel. This is called the hidden layer because this layer retards corrosion of the metal. In addition, manufacturers typically add large amounts of carbon and manganese to give the steel its austenitic character. In addition, this material is more expensive than low alloy steel.
What is the difference between low alloy steel and high alloy steel?
Both low-alloy and high-alloy steel have improved properties than carbon steel. However, the key difference between low alloy steel and high alloy steel is that low alloy steels contain less than 0.25% alloying elements, while high alloy steels contain more than 10% alloying elements. In the chemical composition, low alloy steel contains iron, carbon (less than 0.2%) and other alloying elements such as Nickel (Ni), Chromium (Cr), Molybdenum (Mo), Tungsten (V), Boron (B), Tungsten ( W) and Copper (Cu), while high alloy steel contains iron, chromium, nickel, carbon, manganese, etc.
Conclusion – Low Alloy Steel vs High Alloy Steel
Both low-alloy and high-alloy steel have improved properties than carbon steel. The main difference between low alloy steel and high alloy steel is that low alloy steels contain less than 0.25% alloying elements, whereas high alloy steels have more than 10% alloying elements.
raznisa.ru
Alloying Additives
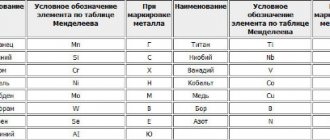
To alloy steels, chemical elements from different groups of the periodic table are used. Alloying metals (in Russian markings of alloys are designated by Russian letters) are introduced into the alloy to change the following characteristics:
- Nickel (N) – increases heat capacity, viscosity, ductility, reduces fragility, which is important for pressure treatment.
- Chrome (X) – increased hardness and impact resistance. Strong protection against corrosion, which is why there is a lot of chromium in stainless steel.
- Niobium (B) – improved acid resistance.
- Cobalt (K) – increased heat resistance, increased impact resistance.
- Copper (D) – increase in strength, but with a slight decrease in viscosity. Used primarily in construction steel.
- Titanium (T) and zirconium (Z) – reduction of grain size. The structure of the alloy becomes homogeneous, which reduces the likelihood of cracks.
- Tungsten (B) and molybdenum (M) - increased strength during heat treatment, resistance to rust.
- Aluminum (Au) – adding resistance to scale at high temperatures.
- Vanadium (F) – improved structure, increased heat resistance.
The list is supplemented by non-metallic additives:
- Manganese (G) – reducing the harmful effects of sulfur, phosphorus and oxygen.
- Silicon (C) – increases strength while maintaining viscosity.
- Selenium (E) – improves fluidity, facilitates machining of steel parts.
- Boron (P) – improvement of microstructure, increase in hardenability.
- Nitrogen (A) – improvement of mechanical properties, used in high-alloy steels.
How to decipher steel grade
Alloy (stainless) steels
, unlike unalloyed ones, have a slightly different designation, since they contain elements that are specially introduced in certain quantities to provide the required physical or mechanical properties. Eg:
- chromium (Cr) increases hardness and strength
- Nickel (Ni) provides corrosion resistance and increases hardenability
- Cobalt (Co) improves heat resistance and increases impact resistance
- Niobium (Nb) helps improve acid resistance and reduces corrosion in welded structures.
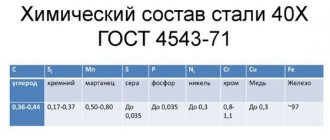
That is why it is customary to include in the names of alloy steels the chemical elements present in the composition and their percentage content. Chemical elements in such steel grades are designated by Russian letters given in the table.
| X-chrome | A-nitrogen |
| C-silicon | N-nickel |
| D-copper | M-molybdenum |
| T-titanium | K-cobalt |
| B-tungsten | B-niobium |
| G-manganese | E-selenium |
| F-vanadium | C-zirconium |
| R-boron | U-aluminum |
There is also a marking H, which tells us that the alloy contains rare earth metals, such as cerium, lanthanum, neodymium and others. Cerium (Ce) affects the strength and ductility of steel, and neodymium (Nd) and lanthanum (La) reduce porosity and sulfur content in steel and refine the grain.
Classification by purpose
Carbon structural steels are characterized by fairly good mechanical properties, which are determined by standard tests, and are characterized by structural strength. They are intended for piece and mass production of welded and prefabricated metal structures and for the production of various machine parts and units.
Among structural carbon steels, it is also customary to distinguish the following grades:
- foundries Used for making castings;
- boiler rooms Their intended purpose is parts and structural parts of boilers, fittings and pressure vessels;
- automatic. They are characterized by improved machinability, are easy to cut and reduce tool wear. Therefore, they are widely used for the automated production of parts on universal and special machines.
Tool carbon steel has a more specific application and is characterized by high hardness, heat resistance and wear resistance. Such grades contain carbon in the range of 0.7...1.5% and can be used for the manufacture of cutting and measuring industrial and laboratory tools, stamping equipment and punches.
Carbon steels of ordinary quality, based on guaranteed characteristics and taking into account the nature of the application, are classified into the following categories: steels of group A (St0...St6) are supplied according to mechanical properties, steels of group B (BSt0...BSt6) - according to chemical composition, steels of group B (VSt1... VSt5) – according to mechanical characteristics and chemical composition.
Alloy tool steels
Alloyed tool steels have GOST 5950–2000. Alloying elements introduced into tool steels increase heat resistance (tungsten, molybdenum, cobalt, chromium), hardenability (manganese), toughness (nickel), wear resistance (tungsten). Compared to carbon alloy tool steels, they have the following advantages :
- good hardenability;
- high ductility in the annealed state;
- significant strength in the hardened state, higher cutting properties.
Low-alloy tool steels contain up to 2.5% alloying elements, have high hardness (HRC 62...69), significant wear resistance, but low heat resistance (200...260°C). They are used to make tools of more complex shapes. In low-alloy steels X, 9ХС, ХВГ, ХВСГ, the main alloying element is chromium . Steel X is alloyed with chromium only. The increased chromium content increases its hardenability. Steel X is calcined in oil completely in a section up to 25 mm, steel U10 - only in a section up to 5 mm.
X steel is used for the manufacture of turning, planing and slotting tools. Steel 9ХС , in addition to chromium, is alloyed with silicon. Compared to steel X, it has greater hardenability - up to 35 mm; increased heat resistance - up to 250...260°C (steel X - up to 200...210°C) and better cutting properties. Drills, reamers, cutters, taps, and dies are made from 9ХС steel. HVG steel is alloyed with chromium, tungsten and manganese ; has hardenability to a depth of 45 mm. HVG steel is used for the production of large and long broaches, long taps, long reamers, etc.
KhVSG steel is a complex alloy and, in comparison with 9ХС and KhVG steels, is better hardened and calcined. When cooled in oil, it is completely calcined in a cross section of up to 80 mm. It is less sensitive to overheating. Its heat resistance is the same as that of 9XC steel. HVSG is used for the manufacture of round dies, reamers, large broaches and other cutting tools.
High-alloy tool steels contain tungsten, chromium and vanadium in large quantities (up to 18% of the main alloying element); have high heat resistance (600...640°C). They are used to manufacture high-performance cutting tools designed for processing high-strength steels and other difficult-to-cut materials. Such steels are called high-speed (GOST 19265–73). High-speed steels are designated by the letter P, the number after which indicates the tungsten content. The content of chromium (4%) and vanadium (2%) in high-speed steel grades is not indicated. Some high-speed steels additionally contain molybdenum, cobalt and large amounts of vanadium. The grades of such steels contain, respectively, the letters M, K, F and numbers indicating their quantity. For the manufacture of measuring instruments, X, HVG and other steels are used, the chemical composition of which is given in GOST 5950–2000.
For a measuring tool, the change in dimensions of a hardened tool over time is of great importance. Therefore, when heat treating a measuring instrument, attention is paid to stabilizing the stress state. This is achieved by low tempering mode - at a temperature of 120...130°C for 15...20 hours and processing at temperatures below zero (down to –60°C).
Cold deformation dies of small size (25...30 mm cross-section), simple shape, working in light conditions, are made from carbon steels U10, UN, U12. Dies with a cross section of 75...100 mm of more complex shape and for more severe working conditions are made from steels of increased hardenability X, KhVG. To produce tools with high hardness and increased wear resistance , as well as low deformability during hardening, steels with high hardenability and wear resistance are used, for example high-chromium steel Kh12F1 (11...12.5% Cr; 0.7...0.9% V).
For tools that are exposed to high impact loads (such as pneumatic chisels, cutting knives for cold metal cutting shears), steels with a lower carbon content and high viscosity are used - 4ХС, 6ХС, 4ХВ2С, etc.
Hot deformation hammer dies are made from steels 5ХНМ, 5ХГМ, 5ХНВ. These steels contain the same amount (0.5...0.6%) of carbon and are alloyed with chromium. This carbon content makes it possible to obtain a fairly high impact strength; chromium increases the strength and hardenability of steels. Nickel is introduced into these steels to increase toughness and improve hardenability. Tungsten and molybdenum increase hardness and heat resistance, reduce brittleness, refine grain, and reduce the tendency of steel to overheat. Manganese, as a cheaper alloying element, is a substitute for nickel. Hammer die steels are characterized by deep hardenability .
Steel grades with special properties
We bring to your attention a wide range of rolled products from alloy steels with special properties: electrical, relay, scale-resistant, stainless, acid-resistant, hard magnetic and soft magnetic steels. Steels acquire “special properties” due to the introduction into them during the manufacturing process of a certain amount of a certain substance, called an alloying element. Alloying elements can be aluminum, manganese, etc.
The range of metal products meets the requirements:
- round rolled products - GOST 2590-88, GOST 7417;
- square rolled products - GOST 2591-88, GOST 8559;
- hexagonal rolled products - GOST 2879-88, GOST 8560;
- forged square and round rods - GOST 1113-88;
- stripes - GOST 103-76, GOST 4405;
- with special surface finishing - GOST 14955.
Comparison of carbon steel with stainless steel
Ability to absorb odors
Carbon steel itself has a pleasant smell (especially when clean and freshly sharpened), but it quickly absorbs foreign odors. If we plan wood with a carbon knife, this is even great, but for cutting food it is not very good: the “aroma” of onions or smoked fish will take a long time to wash off the tool. There are no such problems with stainless steel; it itself is also odorless. For kitchen knives this is a definite plus.
Unalloyed steels - Chemist's Handbook 21
At atmospheric pressure and high temperatures, carbon monoxide is inert to most materials.
Starting from 500-600, carbon monoxide carburizes unalloyed steel without pressure, and with prolonged exposure to it at temperatures above 700 ° C, the steel becomes brittle, and the release of carbon is observed according to the reaction [p.230] Sparks formed during friction of aluminum parts are very dangerous o metal structures (for example, in fans with an aluminum wheel and a casing made of non-alloy steel), they ignite any explosive mixtures. This is explained by their extremely strong heating due to the heat of the exothermic reaction of the reduction of iron oxide with aluminum. Therefore, it is recommended that the casing of explosion-proof fans be made of sheet aluminum, and the wheel, which carries a rotational load and requires great strength, is made of duralumin and only the bearing shaft is made of steel. To prevent rust from entering the fan from the air supply ducts, they are made of aluminum or painted on the inside with oil paint. [p.205] In a hydrogen sulfide-containing environment, the durability of steel is significantly influenced by its hardness, the level of stress acting in the metal and the concentration of hydrogen sulfide. At low voltages, a hydrogen sulfide-containing environment causes the formation of cracks and delaminations in unalloyed steels, oriented along the rolled product parallel to the vector [p.16] Anodic etching is based on the electrochemical dissolution of the metal and the mechanical tearing off of oxides by released oxygen bubbles. Cathodic etching occurs due to electrochemical reduction and mechanical removal of metal oxides by rapidly released hydrogen. This pickling method is used only for unalloyed steels covered with scale. [p.374]
Copper plating of non-alloy steels. For this purpose, you can use a solution of the composition [p.186]
Barton K., Patch V. The influence of the chemical composition of unalloyed steels on the long-term occurrence of atmospheric corrosion. — Proceedings of the III international conference on the problem of CMEA. Warsaw, 1980, p. 157-158. [p.208]
Almost all iron-based alloys can be borated, but it should be taken into account that their chemical composition significantly affects the structure and depth of the layer. In structural unalloyed steels, with increasing carbon content, the thickness of the borated layer decreases and its boundaries with the base are gradually leveled. As the layer increases, carbon is pushed deeper into the sample, since it is almost insoluble in the phases PeB and ResB, and its content at the boundary can be several times higher than the average content in steel. To mitigate this undesirable phenomenon, it is recommended to increase the duration of the process in order to diffusion level the excess carbon concentration. The penetration depth of boron for steel containing 0.28% C at a process temperature of 800° C increases from 25 to 60 μm with an increase in exposure from 1 to 3 hours. An increase in carbon concentration from 0.28 to 0.56% reduces the layer depth to 40 microns. [p.41]
Alloying elements of low-alloy steels during soil corrosion reduce the initial rate of formation of corrosion pits. The maximum pit depth is also less than in non-alloy steels. Chromium and molybdenum increase the corrosion resistance of alloy steels in the presence of colloids. Low-alloy steels are used to make structures for structures located in aggressive soils. [p.91]
B to N - behave like unalloyed steels. [p.245]
Austenitic high-alloy steels B to H - behave like unalloyed steels, [p.245]
Sodium nitrite is used to protect against corrosion of unalloyed steel in contact with stainless steel, as well as nickel-plated and chrome-plated parts. It is also successfully used to protect steel from corrosion that occurs when steel is simultaneously exposed to alkaline water and local stresses, while the sodium nitrite content in the water should be 30-40% of the alkali content. [p.83]
Carbon non-alloy steels 400 Low alloy steels [p.178]
Much of this chapter is devoted to the corrosion behavior of conventional (carbon) unalloyed steel, for two reasons. Firstly, this is the most widely used structural material in marine conditions, and secondly, the factors influencing corrosion have been studied in this case in the most detail. The corrosion rate of unalloyed steel (hereinafter we will simply call it steel) is largely determined by the kinetics of cathodic reduction of oxygen. [p.13]
Data on the corrosion behavior of carbon (unalloyed) steel and low-alloy steels during 8- and 16-year exposure at a depth of 4.3 m in the Pacific Ocean near the Panama Canal Zone are presented in Table. 12 and in Fig. 29-31. Average corrosion rates, calculated from mass loss, for steels containing 2 and 5% N1, are approximately the same as for carbon steel (see Fig. 29), but not [p.51]
In the adsorber, desorber, fans, process control system, and adsorbent conveyors, sulfuric acid and moisture are not released in a free state and there is no threat of corrosion. Therefore, these units are made of non-alloy steel. In the unit for purifying and cooling gas containing sulfur dioxide, as well as in the units for processing sulfur dioxide into commercial products, the use of acid-resistant materials, alloy steels, ceramics, lead, etc. should be provided [p.278]
To determine antimony in unalloyed steels, prepare a solution of 60 g of potassium iodide and 6 g of ascorbic acid in 40 ml of water, transfer the solution to a 100 ml volumetric flask and dilute with water to the mark. Prepare only before use. [p.41]
The iodide method is characterized by a fairly high selectivity (when measuring optical density at 425 nm) and, when using suitable masking reagents, allows the determination of 3b in aluminum alloys [843], cast iron [1185], unalloyed steels [512], copper-tin alloys [1436], alloys of 3b with Au, as well as in tin, lead and copper [1043]. [p.42]
Materials for the manufacture of equipment, suspensions, fixtures, screens. The bath bodies are made of unalloyed steel, the coils and lining of the chrome plating baths are made of lead. [p.225]
To determine phosphorus in unalloyed steels on a medium-dispersion quartz spectrograph, an alternating current arc generator (type DG-2) is usually used [331, 426]. The arc current is 12-14 a, the current in the primary winding of the transformer is 0.4 a. Analytical gap 2.5 mm, auxiliary gap [p.142]
Determination of unalloyed steels by pallium iodide [p.139]
Non-alloy steels do not require special preparation. When preparing low-alloy steels for coating, it should be borne in mind that the presence of chromium and nickel increases the tendency to passivation. Therefore, it is necessary to additionally activate the surface. [p.56]
Soft iron and unalloyed steels are resistant to dry fluorine up to a temperature of 400°C. [p.15]
Non-alloy steels type 22K and 2.9 3 10″ [p.36]
Non-alloy steels type 22K and their welded joints [p.103]
Unalloyed steels type 22K and their welded joints 2.9 3 10-“ [p.186]
Desalting of raw materials. The resin contains salts - sodium phenolate, formed as a result of neutralization of sulfuric acid in the decomposed mass of cumene hydroperoxide with alkali, and iron phenolate - a product of corrosion of equipment made of unalloyed steel. [p.112]
The nature of the change in impact toughness with decreasing temperature varies significantly for different steels (Fig. 45). The greatest decrease in impact strength is observed for carbon steels in the temperature range from -b15 to -40 °C. In this regard, unalloyed steels are usually used only up to temperatures of about -50°C [126]. For use when [p.135]
Diffusion aluminizing (alitizing). Aluminized unalloyed steels are widely used instead of heat-resistant high-alloy steels. [p.106]
Nickel, at those concentrations that are typical for the steels under consideration, tends to somewhat deteriorate the resistance to corrosion resistance. However, large additions of nickel (>8%) in some environments have a positive effect [33]. Thus, the available data suggest that there is a certain critical concentration of nickel, below which the resistance of steel deteriorates with increasing nickel content, and above which it improves. This critical concentration depends on the specific environment and possibly on the strength level of the material. For example, according to available data, with a constant high strength, Kcr decreases; low-strength nickel concentrations of 1.2 and 3.5% N1 impair resistance when tested in hydrogen (see Fig. 5), although the difference from unalloyed steel is small [32] . In the case of nitrate media there is a critical [p.56]
The department studied and summarized the experience in the production of low-manganese and non-alloy steel for shaped castings at the plants of the Ministry of Construction and Dormash and published standard technological instructions. A method for deoxidizing steel with aluminum is proposed by placing cast aluminum rings and alloys on the stopper of a steel-pouring ladle, which can significantly improve the use of aluminum and the completeness of steel deoxidation. This deoxidation method was adopted and implemented at the factories of the Ministry of Construction and Construction, the Ministry of Heavy Machinery, etc. [p.75]
As a material for heat exchangers, low-carbon unalloyed steels with a carbon content of up to 0.25% are used. They are very plastic and therefore can be easily processed by pressure, bending and straightening in a hot and cold state; they weld well; they can also be used in the form of steel shaped castings. At the same time, these steels are characterized by quite satisfactory mechanical properties; they are quite strong at temperatures up to 450°C, are not prone to brittle fracture, and withstand dynamic loads well. [p.18]
To avoid the formation of cracks, metal of large thicknesses is welded with preliminary and accompanying heating. When the vessel wall thickness is less than 16 mm, low-carbon non-alloy steel with a carbon content of up to 6.2% is welded without pre-heating. With a wall thickness of more than 16 mm. preliminary and accompanying heating to 100-200 C is required. [p.141]
According to GOST 11658-65, aluminum in cast iron and non-alloy steel is determined by aluminone without separation. Iron is reduced with ascorbic acid to Fe (I), which does not interfere with the determination of aluminum. In steels that contain titanium and vanadium, this GOST provides for the preliminary removal of iron by extraction with ether and the separation of titanium and vanadium by precipitation in the form of cupferonates, i.e., the same as in the Short method [11621. [p.212]
The surface of the autoclave and the aluminum trial-keel used must be inert with respect to the cutting-stump reaction, i.e., first of all, not contain even traces of nickel compounds. Typically, triethylaluminum distilled under vacuum meets this requirement. It is best if the autoclave is made of unalloyed SM steel, but good results have also been obtained with autoclaves made of VA steel. The internal surfaces must be mechanically well cleaned of all adhering particles by washing with some kind of hydrocarbon. Immediately before carrying out the experiments, it is advisable to treat the autoclave as follows: heat the autoclave while shaking with Vio-/20 volume of triethylaluminum or other trialkyl aluminum (or a solution of aluminum trialkyl in a hydrocarbon) for several hours to 200 °, and then introduce cold ethylene under a pressure of about 60 atm and heat while shaking up to 110° until the pressure stops dropping. After such treatment and cooling, the autoclave should not contain (with the valve open) any significant amount of butylene. Ethylene absorption of up to 2% is considered normal. If a larger amount of butylene has formed, the liquid contents of the autoclave should be removed without access to air and the operation repeated. An autoclave thus treated remains suitable for the reaction as long as it is used only for the extension reaction. [p.181]
Method using sodium pyrrolidine dithiocarbamate. This reagent was proposed as a universal reagent for the extraction-photometric determination of elements of the hydrogen sulfide group [835]. The work [836] describes the determination of arsenic in cast iron and unalloyed steel. Maximum light absorption of chloroform extract t/ms-pyrrolidine dithiocarbamate [p.72]
Sodium pyrrolidinedithiocarbamate is used as a reagent for the extraction-photometric determination of arsenic in cast iron and unalloyed steel [836]. Arsenic(1I) pyrrolidine dithiocarbamate is extracted with chloroform and the optical density of the resulting extract is measured. [p.128]
An oxyquinoline phosphoromolybdate method for the determination of P and Az in unalloyed steels has been proposed [9561. The amount of P and Az is determined by the titrimetric oxyquinoline molybdate method. Phosphorus is determined separately in the form of phosphoromolybdate, and the amount of arsenic is determined by the difference. When steel contains up to 0.001% P and Az, the determination error is + 0.0002 abs.% for P and + 0.0005 abs. % for Az. [p.33]
Chromium steels containing 4-6% Cr are considered semi-heat-resistant. Steels of this class, due to their availability, increased corrosion resistance and strength, are widely used in the oil industry for the manufacture of cracking units. The heat resistance of these steels in air and in flue gases with a significant content of sulfur compounds at temperatures of 500-600 ° C is approximately 3 times higher than the heat resistance of unalloyed steels. [p.171]
The installation equipment is made of ordinary unalloyed steel, since the process takes place at low temperatures and the solvent does not cause corrosion of the equipment. Ease of operation and the possibility of complete automation of the process allow you to reduce labor costs. The cost of the solvent is low, and the service life is long. To heat the streams to the required temperature, crushed steam can be used. [p.144]
O-rings on valves for superheated steam and similar operating conditions almost always have a nitrided surface. When nitriding, a very strong surface is formed. layer with a hardness of about 1000 Brinell, without warping the workpiece. This layer provides exceptionally high abrasion resistance even at temperatures around 500°. The disadvantages of the nitrided layer include its low resistance to corrosion, which is only slightly higher than that of unalloyed steel. In addition, the nitrided surface is quite susceptible to erosion. [p.262]
The use of (NH4)g504 and especially NaHCO3 allows the process to be carried out in a wider range of concentrations of working solutions and a higher range of pH values. In this case, the equipment can be made of non-alloy steel. [p.23]
In the manufacture of boiler inspection objects, the most common material is low-carbon non-alloy steel. They are very plastic and therefore lend themselves well to pressure processing, bending and straightening in hot and cold states, and are well welded. At the same time, these steels are characterized by quite satisfactory mechanical properties; they are strong enough when heated to 450 C, are not prone to brittle fracture, and withstand dynamic loads well. [p.30]
chem21.info
Difference between alloy and unalloy steel
Definition
Alloy Steel: Alloy steel is a type of steel consisting of iron, carbon and some other elements.
Non-Alloy Steel: Non-alloy steel is a type of steel that has no other elements added during melting.
Presence of carbon
Alloy Steel: Alloy steel consists of a large amount of carbon.
Non-alloy Steel: Non-alloy steel has less or no carbon content.
smelting
Alloy Steel: Alloy steel is made by adding various elements during melting.
Types of non-alloy steel
Non-alloy steel can be of the following types:
- For railway casting – these are switches, rails, as well as other products for creating railway tracks. Wheels and axles are also manufactured, that is, all structures that have high reliability requirements;
- Steel 10895 - most often such parts are used in magnetic circuits for electrical devices. This is a fairly ductile steel that can be processed in a hot state;
- Carbon non-alloy steel;
- High carbon steel;
- Medium carbon;
- Low carbon.
Carbon-containing steels are among the most affordable in terms of cost, because their composition is very simple and does not require the use of any expensive ferroalloys.
Examples of marking steels of various types
Determining the grade of steel and assigning an alloy to a certain type is a task that should not cause any problems for a specialist. You don’t always have a table at hand that gives a breakdown of brand names, but the examples given below will help you figure it out.
Content of elements in common steel grades (click to enlarge)
Structural steels that do not contain alloying elements are designated by the letter combination “St”. The numbers following are the carbon content, calculated in hundredths of a percent. Low-alloy structural steels are marked somewhat differently. For example, 09G2S steel contains 0.09% carbon, and alloying additives (manganese, silicon, etc.) are contained within 2.5%. 10KhSND and 15KhSND, which are very similar in their markings, differ in different amounts of carbon, and the share of each alloying element in them is no more than 1%. That is why there are no numbers after the letters indicating each alloying element in such an alloy.
20Х, 30Х, 40Х, etc. – this is how structural alloy steels are marked; the predominant alloying element in them is chromium. The number at the beginning of such a mark is the carbon content in the alloy in question, calculated in hundredths of a percent. The letter designation of each alloying element can be followed by a number, which is used to determine its quantitative content in the alloy. If it is not there, then the specified element in the steel contains no more than 1.5%.
You can consider an example of the designation of chromium-silicon-manganese steel 30KhGSA. According to the labeling, it consists of carbon (0.3%), manganese, silicon, and chromium. It contains 0.8–1.1% of each of these elements.
Features of non-alloy steel products
Each type of steel has its own characteristics and it is important to initially understand under what conditions the casting will be used. This is the only way to create a high-quality, reliable casting that will meet all requirements.
Thus, non-alloy steel is one of the cheapest, but at the same time its quality can ensure reliable operation of many elements. Each grade of unalloyed steel has its own mechanical characteristics, which can be regulated by adjusting the amount of carbon in the metal itself.
If there is little carbon, the steel will be soft, but if hard, strong steel is needed, in addition to carbon, carburization can also be used, that is, the process of heat treatment of metal with a high carbon content.
Source
Alloy and non-alloy steel difference
Any specialist who deals with metal is familiar with the concept of “steel grade”. Deciphering the markings of steel alloys makes it possible to get an idea of their chemical composition and physical characteristics. Understanding this marking, despite its apparent complexity, is quite simple - it is only important to know on what principle it is compiled.
Rarely does production operate without steel, so understanding its grades is extremely important
The alloy is designated by letters and numbers, which can be used to accurately determine which chemical elements it contains and in what quantity. Knowing this, as well as how each of these elements can affect the finished alloy, it is possible to determine with a high degree of probability exactly what technical characteristics are characteristic of a particular grade of steel.
Types of steels and features of their markings
Steel is an alloy of iron and carbon, the content of which is no more than 2.14%. Carbon gives the alloy hardness, but if it is in excess, the metal becomes too brittle.
One of the most important parameters by which steels are divided into different classes is the chemical composition. Among the steels according to this criterion, alloyed and carbon steels are distinguished, the latter are divided into low-carbon (carbon up to 0.25%), medium-carbon (0.25-0.6%) and high-carbon (they contain more than 0.6% carbon).
Types of steels
By including alloying elements in the steel, it can be given the required characteristics.
It is in this way that, by combining the type and quantitative content of additives, grades with improved mechanical properties, corrosion resistance, magnetic and electrical characteristics are obtained.
Of course, it is possible to improve the characteristics of steels using heat treatment, but alloying additives make it possible to do this more efficiently.
Based on the quantitative composition of alloying elements, low-, medium- and high-alloy alloys are distinguished. In the first alloying elements there is no more than 2.5%, in medium alloyed elements - 2.5-10%, in highly alloyed ones - more than 10%.
Properties and types of steels
Steel has the following properties:
- Physical: heat capacity, electrical and thermal conductivity, expansion when heated.
- Mechanical: strength, hardness, elasticity, plasticity, viscosity, endurance.
- Chemical: heat resistance, scale resistance, fire resistance, corrosion resistance.
To significantly change the properties of the alloy, alloying elements - other metals and non-metals - are introduced into the steel. This technology was created back in the 19th century. Steels are called alloyed if the proportion of each element is at least 0.1%.
Chemical composition of alloy steel
There are constant components - these are those that are present in any alloy of this category; there are also optional alloying ingredients. First, we list those that form the classic material:
- Iron. It is a very malleable metal in itself, which is mined from ore. The peculiarity is that there is quite a lot of it in the bowels of the earth; in terms of extraction it is in second place after aluminum. It reacts well, which is why it can be fused in various ways. In percentage terms, it can be from 45 to 97-99 percent. We will not name the exact number of parts, since there are many grades of steel, the composition of which varies.
- Carbon. This is one of the integral components. The combination of these substances increases the natural qualities of iron. On average, it is added from 0.1% to 1.4% to the total mass. The higher its content, the higher the strength. All steel products are divided into carbon and low-carbon.
- Manganese. An interesting ingredient that is also an alloying ingredient. Although if it is less than 1%, then it does not impart any special properties. In itself, it is a very beautiful silvery metal; it is from this that the ingots acquire their characteristic iridescence. But the main merit of manganese is that it is a deoxidizer, that is, it helps remove oxygen from the alloy, which, in turn, negatively affects the properties. There are interesting compounds (named after Hadfield - the creator) that contain about 11 - 14 percent. In this case, the steel loses its magnetic qualities, and also becomes very impact-resistant and wear-resistant, since it is strengthened upon impact.
- Silicon is an essential element, which, with a high content (more than 0.8%), has alloying properties. It is also a deoxidizer, and also increases durability, elastic limit, heat resistance and some other features.
In addition, the composition usually contains harmful and hidden impurities. They are trying to get rid of them, but, unfortunately, they cannot be completely removed. Therefore, in extremely small doses the samples contain:
- Sulfur, which increases red brittleness - cracks appear on the heated workpiece.
- Phosphorus, it leads to an increase in cold brittleness, that is, fragility.
- Oxygen, nitrogen and hydrogen “loose” the structure.
- Oxides and nitrides can lead to tears.
The third group of components are random. They end up in the container along with the charge, that is, with a mixture of starting materials, and do not have a positive effect. They can be harmless or not very useful, but due to the small proportion of content they are practically unimportant. These include:
- copper;
- zinc;
- lead;
- chromium;
- nickel, etc.
And finally, the fourth group is special alloying additives. These elements are additionally introduced to enhance certain characteristics. They are the ones who make the classic alloy hardened. We will list the components in more detail in the corresponding section of the article.
What is steel
An alloy based on iron (at least 45%) is called steel. Depending on the percentage of the second initial component - carbon, alloys are distinguished into high-carbon (0.6-2.14% C), medium-carbon (0.25-0.6% C), and low-carbon (no more than 0.25% C) . The higher this indicator, the more durable and elastic the steel, but at the same time with reduced ductility and impact resistance.
Alloy steel example
The required components of the alloy are deoxidizing agents - manganese and silicon. These chemical elements are present in small quantities and do not affect the properties. Their goal is to neutralize the harmful effects of oxygen.
Even high-quality steel contains harmful impurities that cannot be eliminated. This:
- sulfur, which causes cracks;
- phosphorus, which increases fragility (cold brittleness);
- nitrogen, oxygen, hydrogen – disintegrants of steel structure;
- oxides and nitrides leading to ruptures.
In addition to the listed components, carbon alloys always contain other substances that come along with the starting materials during smelting: copper, zinc, chromium, nickel, lead. The level of their content is so insignificant that they have neither a positive nor a negative effect.
Features of alloy steel - varieties, application
In the modern world there are a large number of varieties of steel. This is one of the most popular materials, which is used in almost all industries.
What is alloy steel
This is carbon steel, to improve its technological properties, special alloying elements have been introduced. The percentage of additives in the composition is small, but even with a small concentration, the physical properties of the metal improve several times.
Depending on the type of additives used in steel production, the metal acquires the following properties:
- resistance to corrosion;
- elasticity;
- infusibility;
- strength.
To impart the listed qualities, the following metals are added to the composition:
- chromium;
- nickel;
- molybdenum;
- tungsten;
- copper.
Often, it is enough to add 1 - 3% alloying elements to carbon steel to give it the necessary properties and qualities.
:
Structural alloy steels
Thick-walled structural steel pipes
The classification of this type of low-carbon iron is quite extensive. Among the parameters that determine the sorting of structural steel are:
- percentage mass of alloying elements;
- chemical composition and base admixture;
- quality of the metal, its surface (two different categories);
Alloy steels
Alloy steels are alloys whose properties are improved by adding additional components called alloying components. Their use is driven by the desire to achieve different properties from the resulting raw materials that are necessary in different situations.
This alloy has increased strength and does not corrode longer. The areas of its application are quite diverse. Basically, these are pipes, parts and other products that will be subject to increased temperature changes during operation.
The composition of ordinary metal includes iron, carbon and various impurities. When doping, as mentioned earlier, other components are added to it, called alloying components. Among them: niobium, chromium, nickel, silicon, vanadium, etc. Aluminum and molybdenum are also often found. To increase the strength of the resulting raw material, titanium is often added.
Tags
Brand of steel Steel casting Unalloyed steel Unalloyed steel represents tool steel also the type of steel produced unalloyed steel Unalloyed steel can Steel 10895 Carbon steels are Brand of steel Unalloyed steel Unalloyed steel Unalloyed steel Types of unalloyed steel Unalloyed steel Unalloyed steel unalloyed steel unalloyed steel
welding temperature working equipment condition
| - a general term for varieties of a group of steels intended for the manufacture of blanks in the form of castings, castings, parts and assemblies for machines and mechanisms. Cast structural steels with a carbon content of up to 0.7% are widely used in mechanical engineering, machine tools for the manufacture of body parts, frames and bases, parts that transmit torques, perform linear movements, and carry tensile, bending and torsion loads. At the same time, steels with a carbon content not exceeding 0.25%, used for building structures, must have high weldability. A distinction is made between carbon steel and high-strength low-alloy steel (less than 5% alloying elements); Structural steel is also distinguished by purpose (steel for the construction of bridges, for elements of high-rise buildings, military and aviation equipment, as well as for the preparation of parts). Structural steels can be obtained by casting into various forms (for example: pulleys, gears, plates, housing parts, etc.) thereby obtaining them ready for use as load-bearing elements of machine structures and independent elements. |
Alloy structural steels
Alloy steels are widely used in tractor and agricultural engineering, in the automotive industry, heavy and transport engineering, and to a lesser extent in machine tool building, tool and other types of industry. This steel is used for heavily loaded metal structures.
Steels in which the total amount of alloying elements does not exceed 2.5% are classified as low-alloy, those containing 2.5-10% are alloyed, and more than 10% are classified as high-alloy (iron content more than 45%).
Low-alloy steels are most widely used in construction, and alloy steels are most widely used in mechanical engineering.
Alloyed structural steels are marked with numbers and letters. The two-digit numbers given at the beginning of the brand indicate the average carbon content in hundredths of a percent; the letters to the right of the number indicate the alloying element. For example, steel 12Х2Н4А contains 0.12% C, 2% Cr, 4% Ni and is considered high quality, as indicated by the letter A at the end of the grade.
Construction low alloy steels
Low alloy steels are those containing no more than 0.22% C and a relatively small amount of non-deficient alloying elements: up to 1.8% Mn, up to 1.2% Si, up to 0.8% Cr and others.
These steels include steels 09G2, 09GS, 17GS, 10G2S1, 14G2, 15HSND, 10KHNDP and many others. Steels in the form of sheets and shaped sections are used in construction and mechanical engineering for welded structures, mainly without additional heat treatment. Low-alloy low-carbon steels are weldable.
For the manufacture of large diameter pipes, 17GS steel is used (0.2=360MPa,b=520MPa).
Reinforcing steels
To reinforce reinforced concrete structures, carbon or low-carbon steel is used in the form of smooth or periodically profiled rods.
Steel St5sp2 – в=50MPa, 0.2=300MPa, =19%.
Cold forming steels
To ensure high formability, the ratio w/0.2steel should be 0.5-0.65 at at least 40%. The more carbon it contains, the worse the stampability of steel is. Silicon, increasing the yield strength, reduces the formability, especially the ability of steel to be drawn. Therefore, for cold stamping, cold-rolled boiling steels 08kp, 08Fkp (0.02-0.04%V) and 08Yu (0.02-0.07%Al) are more widely used.