Aluminum is a chemical element of group III of the periodic table of Mendeleev (atomic number 13, atomic mass 26.98154). In most compounds, aluminum is trivalent, but at high temperatures it can also exhibit the +1 oxidation state. Of the compounds of this metal, the most important is Al2O3 oxide.
Aluminum is a silvery-white metal, lightweight (density 2.7 g/cm3), ductile, a good conductor of electricity and heat, melting point 660oC. It is easily drawn into wire and rolled into thin sheets. Aluminum is chemically active (in air it is covered with a protective oxide film - aluminum oxide. Aluminum oxide (Al2O3) reliably protects the metal from further oxidation. But if aluminum powder or aluminum foil is heated strongly, the metal burns with a blinding flame, turning into aluminum oxide. Aluminum even dissolves in dilute hydrochloric and sulfuric acids, especially when heated. But in highly diluted and concentrated cold nitric acid, aluminum does not dissolve. When aqueous solutions of alkalis act on aluminum, the oxide layer dissolves, and aluminates are formed - salts containing aluminum as part of the anion:
Al2O3 + 2NaOH + 3H2O = 2Na[Al(OH)4].
Aluminum, devoid of a protective film, interacts with water, displacing hydrogen from it:
2Al + 6H2O = 2Al(OH)3 + 3H2
The resulting aluminum hydroxide reacts with excess alkali, forming hydroxoaluminate:
Al(OH)3 + NaOH = Na[Al(OH)4].
The overall equation for the dissolution of aluminum in an aqueous alkali solution has the following form:
2Al + 2NaOH +6H2O = 2Na[Al(OH)4] + 3H2.
Aluminum also actively interacts with halogens. Aluminum hydroxide Al(OH)3 is a white, translucent, gelatinous substance.
The earth's crust contains 8.8% aluminum. It is the third most abundant element in nature after oxygen and silicon and the first among metals. It is part of clays, feldspars, and mica. Several hundred Al minerals are known (aluminosilicates, bauxites, alunites, and others). The most important aluminum mineral, bauxite, contains 28-60% alumina - aluminum oxide Al2O3.
Aluminum in its pure form was first obtained by the Danish physicist H. Oersted in 1825, although it is the most common metal in nature.
Aluminum production is carried out by electrolysis of alumina Al2O3 in molten cryolite NaAlF4 at a temperature of 950oC.
Aluminum is used in aviation, construction, mainly in the form of aluminum alloys with other metals, electrical engineering (a substitute for copper in the manufacture of cables, etc.), food industry (foil), metallurgy (alloying additive), aluminothermy, etc.
Characteristics of aluminum
- Aluminum density - 2.7*103 kg/m3;
- Specific gravity of aluminum - 2.7 g/cm3;
- The specific heat capacity of aluminum at 20oC is 0.21 cal/deg;
- The melting point of aluminum is 658.7oC;
- The specific heat capacity of aluminum melting is 76.8 cal/deg;
- The boiling point of aluminum is 2000oC;
- Relative change in volume during melting (deltaV/V) - 6.6%;
- Coefficient of linear expansion of aluminum (at a temperature of about 20oC): - 22.9 * 106 (1/deg);
- The thermal conductivity coefficient of aluminum is 180 kcal/m*hour*deg;
Aluminum elastic modulus and Poisson's ratio
| Name of material | Young's modulus, kg/mm2 | Shear modulus, kg/mm2 | Poisson's ratio |
| Aluminum bronze, casting | 10500 | 4200 | — |
| Aluminum wire drawn | 7000 | — | — |
| Rolled aluminum | 6900 | 2600-2700 | 0,32-0,36 |
Reflection of light by aluminum
The numbers given in the table show what percentage of light incident perpendicular to the surface is reflected from it.
| Wave name | Wavelength | Light reflection, % |
| Ultraviolet | 1880 | 25 |
| 2000 | 31 | |
| 2510 | 53 | |
| 3050 | 64 | |
| 3570 | 70 | |
| Visible | 5000 | — |
| 6000 | — | |
| 7000 | — | |
| Infrared | 8000 | — |
| 10000 | 74 | |
| 50000 | 94 | |
| 100000 | 97 |
Typical mechanical properties of wrought aluminum alloys.
Typical mechanical properties of wrought aluminum alloys.
(044)490-04-88
Table from “Industrial wrought, sintered and cast aluminum alloys. Help Guide." resp. ed. F. I. Kvasov, I. N. Frilyander.
| Alloy and its condition | Type of semi-finished product | E | σ0.2 | σв | δ | ψ | τcp | HB | σ-1 |
| (kgf/mm2) | % | (kgf/mm2) | |||||||
| ¹ alternating bending based on 5×108 cycles, the rest - based on 2×107 cycles | |||||||||
| Aluminum alloys of low and medium strength | |||||||||
| ADM | Pressed and rolled | 7100 | 3 | 8 | 35 | 80 | 5,5 | 25 | 3,5¹ |
| AD1N | Same | 7100 | 15 | 10 | 6 | 60 | 7,0 | 32 | 5,5¹ |
| AMtsM | Rolled | 7100 | 5 | 13 | 23 | 70 | 8,0 | 30 | 5¹ |
| AMtsP | » | 7100 | 13 | 18 | 10 | 55 | 10 | 40 | 6,5¹ |
| AMtsN | » | 7100 | 18 | 22 | 5 | 50 | 11 | 55 | 7¹ |
| AMg1M | Pressed and rolled | 6900 | 5,0 | 12 | 28 | – | 10 | 30 | 7¹ |
| AMg1N | Rolled | 7000 | 19 | 21 | 5 | – | 12 | 55 | 9,5¹ |
| AMg2M | » | 7000 | 9 | 19 | 23 | 30 | 12,5 | 45 | 11¹ |
| AMg2H2 | » | 7000 | 21 | 25 | 8 | – | 14 | 68 | 12,5¹ |
| AMg2N | » | 7100 | 23 | 28 | 5 | – | 16,5 | 77 | 14¹ |
| AMg3M | » | 7000 | 12 | 23,5 | 22 | – | 15,5 | 58 | 11,5 |
| AMg3H2 | » | 7000 | ≥23 | 27 | 8 | – | 16 | 75 | 13¹ |
| AMg4M | » | 6900 | 14 | 27 | 23 | – | 16 | – | 13,5 |
| AMg4N2 | » | 6900 | 24 | 32 | 12 | – | 19 | – | – |
| AMg5M | » | 6900 | 18 | 30 | 20 | – | 18 | 65 | 14 |
| AMg5N | » | 7000 | 32 | 42 | 10 | – | 22 | 100 | 15,5¹ |
| AMg6M | » | 7000 | 17 | 34 | 20 | 25 | 21 | – | 13 |
| AMg6N | » | 7000 | 28 | 38 | 6 | – | – | – | – |
| AD31T | Pressed | 7100 | 8 | 17 | 20 | – | – | – | 7,0¹ |
| AD31T1 | » | 7100 | 20 | 24 | 10 | – | 16 | 80 | 9 |
| AD33T | » | 7100 | 14 | 24 | 20 | – | 16,5 | 65 | 10,5 |
| AD33T1 | » | 7100 | 27 | 31 | 12 | 25 | 19 | 95 | 11 |
| AD35T | » | 7100 | 18 | 27 | 15 | – | 15,5 | 60 | – |
| AD35T1 | » | 7100 | 28 | 33 | 8 | 35 | 18 | 95 | 11 |
| AVT1 | » | 7100 | 29 | 35 | 12 | 20 | 21 | 95 | 11,5 |
| Medium strength aluminum alloys | |||||||||
| Alloy and its condition | Type of semi-finished product | E | σ0.2 | σв | δ | ψ | τcp | HB | σ-1 |
| (kgf/mm2) | % | (kgf/mm2) | |||||||
| D1T | Stampings | 7100 | 25 | 41 | 15 | 30 | 27 | 110 | 12,5¹ |
| D16T | Rolled | 6900 | 29 | 44 | 19 | – | 28 | 120 | 12,5 |
| D16T1 | » | 6900 | 40 | 45 | 7 | – | 27 | – | 12,5 |
| D16T1N | » | 6900 | 46 | 50 | 5,5 | – | 28,5 | – | 12,5 |
| D16T | Pressed | 7200 | 38 | 52 | 12 | 15 | 30 | 130 | 14¹ |
| D19T | Rolled | 6900 | 30 | 44 | 20 | – | – | – | |
| D19TN | » | 6900 | 36 | 48 | 13 | – | – | – | |
| D19T | Pressed | 7200 | 34 | 46 | 12 | – | 29 | 120 | |
| M40T | Rolled | 7000 | 25 | 39 | 18 | – | – | – | |
| » | Pressed | 7100 | 31 | 41 | 12 | 17 | – | – | |
| VAD1T | Rolled | 6900 | 28 | 44 | 18 | – | – | – | 12 |
| » | Pressed | 7200 | 36 | 50 | 13 | – | – | – | 14 |
| D20T1 | Rolled | 6900 | 30 | 42 | 11 | 26 | 10,5¹ | ||
| D29T1N | » | 6900 | 36 | 45 | 10 | 29 | 10,5¹ | ||
| D20T1 | Pressed | 6900 | 28 | 42 | 10 | 35 | 27 | 100 | 13 |
| D21T1 | Forgings and stampings | 7000 | 35 | 43 | 9 | 18 | |||
| 1201 | Rolled | 30 | 42 | 12 | |||||
| 1205 | Rolled | 40 | 50 | 9 | |||||
| VD17T1 | Stampings: | 7200 | 34 | 52 | 17 | 20 | 115 | 16 | |
| longitudinal direction | |||||||||
| transverse direction | 7200 | 30 | 44 | 17 | 20 | ||||
| V92T1 | Rolled | 6900 | 30 | 40 | 10 | ||||
| Pressed | 7000 | 34 | 45 | 10 | 11 | 15 | |||
| 1915T1 | Rolled | 6800 | 28 | 36 | 11 | ||||
| Pressed | 7000 | 32 | 38 | 10 | |||||
| 1420T1 | Rolled | 7500 | 27 | 44 | 9 | ||||
| Pressed | 7600 | 31 | 46 | 9 | 12 | ||||
| 1911T1 | Rolled | 6800 | 35 | 42 | 12 | ||||
| Pressed | 7000 | 42 | 50 | 15 | |||||
© "M-Komplekt" 2007 - 2017
www.metmk.com.ua
Aluminum oxide Al2O3
Aluminum oxide Al2O3, also called alumina, occurs in nature in crystalline form, forming the mineral corundum. Corundum has very high hardness. Its transparent crystals, colored red or blue, represent the precious stones ruby and sapphire. Currently, rubies are produced artificially by alloying with alumina in an electric furnace. They are used not so much for decoration as for technical purposes, for example, for the manufacture of parts for precision instruments, watch stones, etc. Ruby crystals containing a small admixture of Cr2O3 are used as quantum generators - lasers that create a directed beam of monochromatic radiation.
Corundum and its fine-grained variety containing a large amount of impurities - emery, are used as abrasive materials.
Methods of obtaining
Aluminum oxide can be obtained by various methods:
1. Combustion of aluminum in air:
4Al + 3O2 → 2Al2O3
2. Decomposition of aluminum hydroxide when heated:
2Al(OH)3 → Al2O3 + 3H2O
3. Aluminum oxide can be obtained by decomposition of aluminum nitrate:
4Al(NO3)3 → 2Al2O3 + 12NO2 + 3O2
Chemical properties
Aluminum oxide is a typical amphoteric oxide. Interacts with acidic and basic oxides, acids, alkalis.
1. When aluminum oxide interacts with basic oxides, aluminate salts are formed.
For example, aluminum oxide reacts with sodium oxide:
Na2O + Al2O3 → 2NaAlO2
2. Aluminum oxide interacts with soluble bases (alkalis). In this case, aluminate salts are formed in the melt, and complex salts are formed in the solution. In this case, aluminum oxide exhibits acidic properties.
For example, aluminum oxide reacts with sodium hydroxide in the melt to form sodium aluminate and water:
2NaOH + Al2O3 → 2NaAlO2 + H2O
Aluminum oxide dissolves in excess alkali to form tetrahydroxyaluminate:
Al2O3 + 2NaOH + 3H2O → 2Na[Al(OH)4]
3. Aluminum oxide does not react with water.
4. Aluminum oxide reacts with acidic oxides (strong acids). In this case, aluminum salts are formed. In this case, aluminum oxide exhibits basic properties.
For example, aluminum oxide reacts with sulfur(VI) oxide to form aluminum sulfate:
Al2O3 + 3SO3 → Al2(SO4)3
5. Aluminum oxide reacts with soluble acids to form medium and acid salts.
For example, aluminum oxide reacts with sulfuric acid:
Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O
6. Aluminum oxide exhibits weak oxidizing properties.
For example, aluminum oxide reacts with calcium hydride to produce aluminum, hydrogen, and calcium oxide:
Al2O3 + 3CaH2 → 3CaO + 2Al + 3H2
Electric current reduces aluminum from oxide (aluminum production):
2Al2O3 → 4Al + 3O2
7. Aluminum oxide - solid, non-volatile. Consequently, it displaces more volatile oxides (usually carbon dioxide) from the salts during fusion.
For example, from sodium carbonate:
Al2O3 + Na2CO3 → 2NaAlO2 + CO2
Question 7. Aluminum alloys, and their composition, properties and operating features
For building structures, aluminum alloys containing alloying components and impurities of 5-7% are used (technical aluminum with impurities up to 1% is used very rarely due to its low strength and only for decorative and enclosing elements). Aluminum alloys are divided into deformable
(processed
by pressure
: pressing, drawing, rolling, stamping, etc.), used in building structures, and in
foundries
, used mainly in mechanical engineering.
Aluminum alloys are alloyed
manganese, magnesium, silicon, zinc, copper, chromium, titanium, or several of these components at the same time, depending on which the alloy system receives a name and brand with a symbol.
Aluminum alloys are supplied in various thermal
processing and hardening (hardening, drawing).
Technical aluminum
has very high corrosion resistance, but is low-strength and ductile.
Aluminum-manganese and aluminum-magnesium
the alloys have high corrosion resistance, relatively high strength and are easy to weld.
Multicomponent alloys
have medium and high corrosion resistance, medium and high strength indicators and can be used in welded and riveted load-bearing and enclosing structures.
To improve corrosion resistance, aluminum alloys can be clad
(coated with a thin film of pure aluminum during the manufacture of the semi-finished product).
The structure of aluminum alloys consists of aluminum crystals strengthened by alloying elements (alloying elements enter into solid solution with aluminum and strengthen it).
In Fig. Figure 1 shows diagrams of the tensile performance of some aluminum alloys (for comparison, a curve for steel 3 is also given).
Picture 1
1-technical aluminum AD1M;
2- alloy 1915T; 3- steel 3 The mechanical properties
of aluminum alloys depend not only on the chemical composition, but also on the conditions of their processing. Aluminum alloys have a tensile elasticity modulus E = 0.7∙104 kN/cm2, and a shear elasticity modulus G = 0.27∙104 kN/cm2, which is almost 3 times less than that of steel; therefore, at equal stresses, the deflections of aluminum structures are 3 times greater. Poisson's ratio =0.3. There is no yield plateau on the stress-strain diagram of aluminum alloys. The yield strength is conventionally taken to be the stress at which the relative residual deformation reaches =0.2%. At temperatures above 100 °C there is a slight decrease in strength characteristics, and starting from about 200 °C creep appears. The coefficient of thermal expansion of aluminum = 0.000023, which is 2 times greater than that of steel. At lower temperatures, all mechanical properties of aluminum alloys improve. The impact strength of alloys at normal temperatures is lower than that of steel (about 3.0 kg∙m/cm2), and almost does not decrease at subzero temperatures.
The change in the mechanical properties of aluminum alloys during aging occurs more intensely than that of steel, and the increase in yield strength and strength is much higher. The increase in the strength of aluminum alloys during aging is taken into account when assigning their design resistances. Calculation formulas for aluminum structures under various force influences have the same form as for steel structures. The values of various coefficients are taken depending on the grades of alloys according to the design standards for aluminum structures SNiP II-24-74.
The advantages of aluminum alloys
include: relatively high strength with low density of the material itself; high manufacturability when processed by pressing, rolling or forging, allowing the production of products of complex shapes; high resistance to corrosion, high mechanical characteristics at low temperatures; no sparking during impacts.
Disadvantages of aluminum alloys:
relatively small elastic modulus; high coefficient of thermal expansion; relative complexity of making connections; scarcity and still high cost; low fire resistance.
Aluminum alloy profiles for aluminum structures are produced by rolling, pressing or bending
. Only flat profiles are rolled: sheets, strips, tapes. Pressed profiles can be of very different shapes; their cross-section must fit into a circle with a matrix diameter of 320 mm (there are separate presses with a matrix diameter of 530 mm). These profiles are made on special presses. A cylindrical billet of aluminum alloy heated to approximately 400°C is pressed through a steel matrix with a hole in the shape of the profile section. The matrix is held by a holder. Both solid and hollow (tubular) profiles can be pressed.
Bent profiles are made by bending thin sheets or strips on roller bending machines or press brakes.
QUESTION 8.
Basics of calculation of metal structures.
Design diagram, supporting fastening of elements. Limit states. Groups of limit states. Calculation of structures based on permissible stresses and comparison with calculations based on limit states Basics of calculation of metal structures
The purpose and purpose of structural calculations is
checking the strength, stability and rigidity of the pre-planned structural design of the structure, which makes it possible to clarify the dimensions and ensure the reliability of the structure at the lowest possible cost of metal. Calculation of structures and their structural elements is carried out on the basis of methods of strength of materials and structural mechanics. The main goal of these methods is to determine the internal forces that arise in structures under the influence of applied loads.
The calculation begins with drawing up a design diagram of the structure, temporarily distracting from the actual cross-sectional shape of the elements. The supporting fastenings of the elements are endowed with some theoretical properties (hinged supports, supports with elastic and rigid pinches, etc.). Having determined the forces in the elements according to the accepted design scheme, they select sections, check the load-bearing capacity and design the fastenings in such a way as to satisfy the assigned tasks. Sometimes more accurate methods for determining stresses are necessary, taking into account the development of plastic deformations. However, the mathematical complexity of these methods forces us to often use a number of coefficients in the formulas, the values of which are given in the tables. According to SNiP II-23-81*, building structures are designed to withstand other force impacts based on limit states.
Beyond the limit state
a state of the structure is accepted in which it ceases to satisfy the operational requirements imposed on it, i.e. either loses its ability to resist external influences, or suffers unacceptable deformation or local damage.
studfiles.net
Aluminum hydroxide
Methods of obtaining
1. Aluminum hydroxide can be obtained by the action of ammonia solution of aluminum salt.
For example, aluminum chloride reacts with aqueous ammonia to form aluminum hydroxide and ammonium chloride:
AlCl3 + 3NH3 + 3H2O = Al(OH)3 + 3NH4Cl
2. By passing carbon dioxide, sulfur dioxide or hydrogen sulfide through a solution of sodium tetrahydroxyaluminate:
Na[Al(OH)4] + CO2 = Al(OH)3 + NaHCO3
To understand how this reaction proceeds, you can use a simple technique: mentally break down the complex substance Na[Al(OH)4] into its component parts: NaOH and Al(OH)3. Next, we determine how carbon dioxide reacts with each of these substances and record the products of their interaction. Because Al(OH)3 does not react with CO2, then we write Al(OH)3 on the right without change.
3. Aluminum hydroxide can be obtained by the action of a lack of alkali on an excess of aluminum salt.
For example, aluminum chloride reacts with a lack of potassium hydroxide to form aluminum hydroxide and potassium chloride:
AlCl3 + 3KOH(insufficient) = Al(OH)3↓+ 3KCl
4. Also, aluminum hydroxide is formed by the interaction of soluble aluminum salts with soluble carbonates, sulfites and sulfides. Aluminum sulfides, carbonates and sulfites are irreversibly hydrolyzed in aqueous solution.
For example: aluminum bromide reacts with sodium carbonate. In this case, a precipitate of aluminum hydroxide precipitates, carbon dioxide is released and sodium bromide is formed:
2AlBr3 + 3Na2CO3 + 3H2O = 2Al(OH)3↓ + 3CO2↑ + 6NaBr
Aluminum chloride reacts with sodium sulfide to form aluminum hydroxide, hydrogen sulfide and sodium chloride:
2AlCl3 + 3Na2S + 6H2O = 2Al(OH)3 + 3H2S↑ + 6NaCl
Chemical properties
1. Aluminum hydroxide reacts with soluble acids. In this case, medium or acidic salts are formed, depending on the ratio of reagents and the type of salt.
For example, aluminum hydroxide reacts with nitric acid to form aluminum nitrate:
Al(OH)3 + 3HNO3 → Al(NO3)3 + 3H2O
Al(OH)3 + 3HCl → AlCl3 + 3H2O
2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H2O
Al(OH)3 + 3HBr → AlBr3 + 3H2O
2. Aluminum hydroxide reacts with acidic oxides of strong acids.
For example, aluminum hydroxide reacts with sulfur (VI) oxide to form aluminum sulfate:
2Al(OH)3 + 3SO3 → Al2(SO4)3 + 3H2O
3. Aluminum hydroxide reacts with soluble bases (alkalis). In this case, aluminate salts are formed in the melt, and complex salts are formed in the solution. In this case, aluminum hydroxide exhibits acidic properties.
For example, aluminum hydroxide reacts with potassium hydroxide in a melt to form potassium aluminate and water:
2KOH + Al(OH)3 → 2KAlO2 + 2H2O
Aluminum hydroxide dissolves in excess alkali to form tetrahydroxyaluminate:
Al(OH)3 + KOH → K[Al(OH)4]
4. Aluminum hydroxide decomposes when heated:
2Al(OH)3 → Al2O3 + 3H2O
A video of the interaction of aluminum hydroxide with hydrochloric acid and alkalis (amphoteric properties of aluminum hydroxide) can be viewed here.
Mechanical properties of aluminum alloys
Collapsing strength of aluminum alloys
Aluminum's collapse strength is also difficult to determine, test, and relate to conventional strength properties as it is for other metals. Collapse is often an important criterion for structures using rivet and bolt connections and therefore “collapse strength” is a widely recognized characteristic. Bearing strength is very loosely defined as the pressure (per unit effective bearing area) applied by a pin in a circular hole. This hole is preliminarily expanded to 2% of the original diameter (Figure 1). This strength for most aluminum alloys is 1.8 times the tensile strength (tensile strength) (Figure 2).
Picture 1
Figure 2
Shear strength of aluminum alloys
The loading diagram for the shear test is shown in Figure 3. For wrought aluminum alloys, the ratio of shear strength to tensile strength varies depending on the chemical composition and manufacturing method from 0.5 to 0.75 (see Figure 2). In the absence of data on shear strength, it is usually taken as 0.55 of the tensile strength.
Figure 3
Rivets made from aluminum grades and Al-Mn alloys (3xxx series) are produced by cold deformation methods to achieve shear strength of up to 200 MPa. Rivets from thermally hardenable alloys are manufactured in an annealed state, then immediately subjected to hardening and natural aging to achieve a shear strength of up to 260 MPa.
The resistance of a material to local plastic deformation that occurs when a harder body, an indenter, is introduced into it is an approximate indicator of the condition of the alloy and is therefore widely used in product control. For aluminum alloys, the Brinell (steel ball), Vickers (diamond pyramid) and Shore (falling diamond cone) methods are used. Brinell hardness ranges from 20 for pure aluminum to 175 for heat-strengthened 7075 alloy (see Figure 2). According to hardness readings, as a rule, their tensile strength is not calculated, as is usually done for steels, since for aluminum alloys the ratio of these two characteristics is far from constant.
Aluminum salts
Aluminum nitrate and sulfate
When heated, aluminum nitrate decomposes into aluminum oxide, nitrogen oxide (IV) and oxygen:
4Al(NO3)3 → 2Al2O3 + 12NO2 + 3O2
Aluminum sulfate, when heated strongly, decomposes in a similar way - into aluminum oxide, sulfur dioxide and oxygen:
2Al2(SO4)3 → 2Al2O3 + 6SO2 + 3O2
Complex aluminum salts
To describe the properties of complex aluminum salts - hydroxyaluminates, it is convenient to use the following technique: mentally break tetrahydroxoaluminate into two separate molecules - aluminum hydroxide and alkali metal hydroxide.
For example, we break sodium tetrahydroxyaluminate into aluminum hydroxide and sodium hydroxide:
We break Na[Al(OH)4] into NaOH and Al(OH)3
The properties of the entire complex can be determined as the properties of these individual compounds.
Thus, aluminum hydroxo complexes react with acidic oxides.
For example, the hydroxo complex is destroyed by excess carbon dioxide. In this case, NaOH reacts with CO2 to form an acid salt (with an excess of CO2), and amphoteric aluminum hydroxide does not react with carbon dioxide, therefore, it simply precipitates:
Na[Al(OH)4] + CO2 → Al(OH)3↓ + NaHCO3
Similarly, potassium tetrahydroxyaluminate reacts with carbon dioxide:
K[Al(OH)4] + CO2 → Al(OH)3 + KHCO3
By the same principle, tetrahydroxoaluminates react with sulfur dioxide SO2:
Na[Al(OH)4] + SO2 → Al(OH)3↓ + NaHSO3
K[Al(OH)4] + SO2 → Al(OH)3 + KHSO3
But under the influence of an excess of strong acid, the precipitate does not form, because amphoteric aluminum hydroxide reacts with strong acids.
For example, with hydrochloric acid:
Na[Al(OH)4] + 4HCl(excess) → NaCl + AlCl3 + 4H2O
True, under the influence of a small amount (lack) of strong acid, a precipitate will still form; there will not be enough acid to dissolve aluminum hydroxide:
Na[Al(OH)4] + НCl(deficiency) → Al(OH)3↓ + NaCl + H2O
Similarly, with a lack of nitric acid, aluminum hydroxide precipitates:
Na[Al(OH)4] + HNO3(deficiency) → Al(OH)3↓ + NaNO3 + H2O
The complex is destroyed by interaction with chlorine water (aqueous solution of chlorine) Cl2:
2Na[Al(OH)4] + Cl2 → 2Al(OH)3↓ + NaCl + NaClO + H2O
In this case, chlorine is disproportionate.
The complex can also react with excess aluminum chloride. In this case, a precipitate of aluminum hydroxide precipitates:
AlCl3 + 3Na[Al(OH)4] → 4Al(OH)3↓ + 3NaCl
If you evaporate water from a solution of a complex salt and heat the resulting substance, you will be left with the usual aluminate salt:
Na[Al(OH)4] → NaAlO2 + 2H2O↑
K[Al(OH)4] → KAlO2 + 2H2O
Hydrolysis of aluminum salts
Soluble aluminum salts and strong acids are hydrolyzed at the cation. Hydrolysis proceeds stepwise and reversibly, i.e. a little:
Stage I: Al3+ + H2O = AlOH2+ + H+
Stage II: AlOH2+ + H2O = Al(OH)2+ + H+
Stage III: Al(OH)2+ + H2O = Al(OH)3 + H+
However, sulfides, sulfites, aluminum carbonates and their acid salts are hydrolyzed irreversibly, completely, i.e. do not exist in an aqueous solution, but are decomposed by water:
Al2(SO4)3 + 6NaHSO3 → 2Al(OH)3 + 6SO2 + 3Na2SO4
2AlBr3 + 3Na2CO3 + 3H2O → 2Al(OH)3↓ + CO2↑ + 6NaBr
2Al(NO3)3 + 3Na2CO3 + 3H2O → 2Al(OH)3↓ + 6NaNO3 + 3CO2↑
2AlCl3 + 3Na2CO3 + 3H2O → 2Al(OH)3↓ + 6NaCl + 3CO2↑
Al2(SO4)3 + 3K2CO3 + 3H2O → 2Al(OH)3↓ + 3CO2↑ + 3K2SO4
2AlCl3 + 3Na2S + 6H2O → 2Al(OH)3 + 3H2S↑ + 6NaCl
You can read more about hydrolysis in the corresponding article.
Aluminates
Salts in which aluminum is an acidic residue (aluminates) are formed from aluminum oxide when fused with alkalis and basic oxides:
Al2O3 + Na2O → 2NaAlO2
To understand the properties of aluminates, it is also very convenient to break them down into two separate substances.
For example, we mentally divide sodium aluminate into two substances: aluminum oxide and sodium oxide.
We break NaAlO2 into Na2O and Al2O3
Then it will become obvious to us that aluminates react with acids to form aluminum salts:
KAlO2 + 4HCl → KCl + AlCl3 + 2H2O
NaAlO2 + 4HCl → AlCl3 + NaCl + 2H2O
NaAlO2 + 4HNO3 → Al(NO3)3 + NaNO3 + 2H2O
2NaAlO2 + 4H2SO4 → Al2(SO4)3 + Na2SO4 + 4H2O
Under the influence of excess water, aluminates transform into complex salts:
KAlO2 + H2O = K[Al(OH)4]
NaAlO2 + 2H2O = Na[Al(OH)4]
Binary compounds
Aluminum sulfide under the action of nitric acid is oxidized to sulfate:
Al2 S3 + 8HNO3 → Al2(SO4)3 + 8NO2 + 4H2O
or to sulfuric acid (under the influence of hot concentrated acid):
Al2 S3 + 30HNO3(conc.hor.) → 2Al(NO3)3 + 24NO2 + 3H2SO4 + 12H2O
Aluminum sulfide decomposes with water:
Al2S3 + 6H2O → 2Al(OH)3↓ + 3H2S↑
Aluminum carbide also decomposes with water when heated into aluminum hydroxide and methane:
Al4C3 + 12H2O → 4Al(OH)3 + 3CH4
Aluminum nitride decomposes under the action of mineral acids into aluminum and ammonium salts:
AlN + 4HCl → AlCl3 + NH4Cl
Aluminum nitride also decomposes under the influence of water:
AlN + 3H2O → Al(OH)3↓ + NH3
Properties of winged metal
Aluminum has the unlucky number 13 in the periodic table. However, this did not affect the happy fate of the metal.
This lightweight silver metal can be easily machined and cast, and has great ductility.
A rare ability - to quickly form oxide films on the surface of pure metal. But these films do not protect against corrosion very well. Chemical and electrochemical oxidation is more reliable. The formula of the oxide film is A12Oz.
Chemical and physical characteristics of aluminum:
- density 2.7 g/cm3;
- melting point 660°C;
- non-ferrous metal boils at a temperature of 2518°C;
- the structure of the crystal lattice is face-centered, cubic;
- oxidation state 0; +3.
With the help of metallic aluminum (its interaction with metal oxides), difficult-to-reduce metals are obtained. This method is called aluminothermy.
| Properties of the atom | |
| Name, symbol, number | Aluminum (Al), 13 |
| Group, period, block | 13, 3, |
| Atomic mass (molar mass) | 26.9815386(8)[1] a. e.m. (g/mol) |
| Electronic configuration | [Ne] 3s2 3p1 |
| Electrons by shell | 2, 8, 3 |
| Atomic radius | 143 pm |
| Chemical properties | |
| Covalent radius | 121 ± 4 pm |
| Van der Waals radius | 184 pm |
| Ion radius | 51 (+3e) pm |
| Electronegativity | 1.61 (Pauling scale) |
| Electrode potential | −1.66 V |
| Oxidation states | 0; +3 |
| Ionization energy | 1st: 577.5 (5.984) kJ/mol (eV) 2nd: 1816.7 (18.828) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Thermodynamic phase | solid |
| Density (at normal conditions) | 2.6989 g/cm³ |
| Melting temperature | 660 °C, 933.5 K |
| Boiling temperature | 2518.82 °C, 2792 K |
| Ud. heat of fusion | 10.75 kJ/mol |
| Ud. heat of vaporization | 284.1 kJ/mol |
| Molar heat capacity | 24.35[2] 24.2[3] J/(K mol) |
| Molar volume | 10.0 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic face-centered |
| Lattice parameters | 4.050 Å |
| Debye temperature | 394 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 237 W/(m K) |
| Sound speed | 5200 m/s |
| CAS number | 7429-90-5 |
Aluminum has one stable isotope, 27Al.
Microstructure of aluminum on the etched surface of the ingot, purity 99.9998%, visible sector size about 55×37 mm.
Not true, but well thought out
There is a story circulating in print media, and now on the Internet, about a peasant who had “seditious conversations about flying to the moon.” A peasant (or tradesman), according to some information from Petrov, according to others from Nikiforov, was exiled to the Kyrgyz village of Baikonur" Allegedly, news of the fact was published in the Moscow provincial news" in 1848. Now, when more than a dozen satellites and stations have gone into space from the Baikonur cosmodrome, this fact looks prophetic and mystical.
But this is not true. Meticulous readers rummaged through the files of this newspaper, and did not find such a note. It's just a beautiful legend.
Aluminum alloys, pros and cons
Code symbol indicating that aluminum can be recycled.
It is not practical to use pure aluminum in building structures. Its strength characteristics are “so-so”. But aluminum alloys are a different matter. About 60 alloys are now known and used. You can choose for any need, for every taste.
Classification of alloys is carried out according to composition, properties, and ability to heat treatment.
Additives of copper, magnesium and manganese, zinc significantly improve the characteristics of the alloy in comparison with pure metal. Aluminum is most often alloyed with these metals. Titanium, lithium, vanadium, cerium, scandium, and some rare earth elements are used less frequently for alloying, but the properties of these alloys are also in demand in industry.
Duralumin
Duralumins are alloys of aluminum with copper (4%), magnesium (0.5%) and small amounts of iron, manganese, and silicon. The disadvantage of duralumin is its susceptibility to corrosion; it is dealt with using anodizing, plating, aviation primer, and painting.
Demanded properties of the alloy: good static and fatigue strength, high fracture toughness.
Widely used in parts and structures where the mass of the product plays an important role. The main consumers of the alloy are aviation, shipbuilding, and astronautics.
For the curious: duralumin was invented in 1909. The “dad” of the alloy is A. Wilm.
Alloy 7075
It was developed by Sumitomo Metal Corporation (Japan) in the strictest confidence.
It is a compound of aluminum with zinc (up to 6%), magnesium (2-2.5%), copper (up to 1.5%). Titanium, silicon, manganese, chromium, and iron are added to the same alloy. These additives amount to no more than 0.5%, but they contribute to the properties of the alloy.
The alloy is comparable in strength to steel, but is three times lighter.
Alloy modifications:
- 7075-0;
- 7075-06;
- 7075-T651;
- 7075-T7;
- 7075-ASR.
The alloys are resistant to corrosion and can be polished well.
Used in the production of rifles for the army and citizens. The automotive, aviation, and marine industries actively use the alloy. Its disadvantage is the rather high price.
There are many different alloys
There are quite a lot of alloys in Russia with different properties:
- D1, D16, 1161, 1163 - aluminum, magnesium, copper;
- AMG1 - AMG6, aluminum and magnesium alloy;
- AD31, AD33, AD35, AB - aluminum, silicon, magnesium. The list is easy to continue.
Old age is a joy
Old age is not always a bad thing. Metal is like a person or wine; with age, the properties of aluminum change; he gets better, stronger, stronger.
Natural aging of metal occurs under normal conditions; we can say that the metal is “ripening.”
Artificial aging occurs during heat treatment and plastic deformation.
There are different types of heat treatment. The choice depends on the purpose of the future alloy.
| Type of heat treatment | What does heat treatment provide? |
| Hardening with full artificial aging | High strength of the alloy, but some reduction in ductility |
| Hardening with stabilizing aging | Good strength, fairly high structural stability |
| Hardening followed by softening tempering | Good ductility, but reduced alloy strength |
| Artificial aging | Increases the strength of the alloy, improves machinability |
| Annealing | Increasing ductility, reducing residual stresses of the metal |
| Hardening | Improves strength characteristics |
| Hardening and incomplete artificial aging | Increases strength while maintaining ductility |
Mechanical properties of aluminum alloys Amg, Amts
The mechanical properties of AMts aluminum alloy depend on the hot rolling temperature.
Increasing the rolling temperature reduces the tensile strength and increases the tensile strength. This relationship is true for semi-finished products in any state: hot-rolled, cold-rolled and annealed. Mechanical properties of AMts sheets in the hot-rolled and annealed state after cold rolling, reduction 80%
| State | Hot rolling temperature, °C | |||
| 480 — 500 | 350 — 380 | |||
| σв, MPa | δ, % | σв, MPa | δ, % | |
| Hot rolled | 157 | 19,3 | 204 | 12,7 |
| Annealed at T, °C: | ||||
| 350 | 110 | 21,0 | 200 | 9,0 |
| 400 | 110 | 22,0 | 160 | 12,0 |
| 500 | 110 | 23,0 | 130 | 19,0 |
Guaranteed mechanical characteristics of semi-finished products from AMts alloy
| Semi-finished products | State | σв, MPa | δ, % | τav, MPa |
| no less | ||||
| Sheets thickness, mm: | M | |||
| 0,3–3,0 | 100–150 | 22 | – | |
| 3,0–6,0 | 100–150 | 20 | – | |
| 0,3–6.5 | H2 (P) | 150–220 | 6 | – |
| 0,3–0,5 | N | 190 | 1 | – |
| 0,5–0,8 | 190 | 2 | – | |
| 0,8–1,2 | 190 | 3 | 30 | |
| 1,2–1,6 | 190 | 4 | 40 | |
| Pipes of all sizes | M | 130 | – | – |
| N | 140 | – | – | |
| Profiles of all sizes | M | 170 | 16 | 160 |
| Rods | GP | 170 | 16 | – |
| Wire for rivets | Without maintenance | – | – | 70 |
| Plates 11–25mm thick | GK | 120 | 15 | – |
Aluminum alloys with magnesium (manganalia) are not strengthened by heat treatment. In industry, a large group of alloys of the Al-Mg system is used: AMg1, AMg2, AMg3, AMg4, AMg5, AM6, AMg61, etc. Semi-finished products from these alloys have high ductility and low strength compared to thermally hardenable alloys such as D16 or V95. Manganalia can be welded well with all types of welding. They are resistant to corrosion in the marine atmosphere.
The strength of aluminum alloys with magnesium Al-Mg is increased by cold-working of semi-finished products: temporary tensile strength and yield strength increase, while ductility decreases. The degree of cold hardening of 35% does not reduce the high corrosion resistance of AMG alloys and does not affect weldability. Due to heating during welding, the heat-affected zone of AMG alloys has the characteristics of an annealed material. An increase in the magnesium content in alloys to 6% leads to a sharp increase in strength characteristics, especially the yield strength. After Mg concentrations above 5.5% (AMg6), the increase in the yield strength slows down significantly. Ductility decreases to 4% magnesium and then slowly increases.
Manganalia retain high corrosive properties under any heating conditions if the magnesium content does not exceed 4.5%. In alloys with 5-7% Mg, the β-phase Al3Mg2 is released along the grain boundaries during quenching and aging, which creates local foci of corrosion. Continuous precipitation of the β-phase is prevented by annealing at 310-325°C, at which the β-phase disintegrates evenly throughout the grain. This structure is etched evenly in the electrolyte.
Alloys AMg4, AMg5, AM6, AMg61 are the most durable alloys of the aluminum-magnesium system.
They have high technological plasticity, but quickly work harden during cold deformation, as well as high values of σв and σ0.2. Guaranteed (at least) mechanical properties of rolled semi-finished products from alloys of the Al-Mg system
| Alloy | State | Semi | Thickness, mm | σв | σ0.2 | δ, % | |
| MPa | |||||||
| AMg2 | M | Sheets | 0,5–1,0 | 165 | – | 16 | |
| 1,0–10,5 | 165 | – | 18 | ||||
| H2 | 0,5–1,0 | 235–314 | 145 | 5 | |||
| 1,0–5,0 | 235–314 | 145 | 6 | ||||
| 5,0–10,0 | 225 | 135 | 6 | ||||
| N | 0,5–1,0 | 265 | 215 | 3 | |||
| 1,0–10,5 | 265 | 215 | 4 | ||||
| GK, without maintenance | 5,0–10,5 | 175 | – | 7 | |||
| Plates | 11,0–25,0 | 175 | – | 7 | |||
| 25,0–80,0 | 155 | – | 6 | ||||
| AMg3 | M | Sheets | 0,5–0,6 | 195 | 90 | 15 | |
| 0,6–5,5 | 135 | 100 | 15 | ||||
| 4,5–10,5 | 185 | 80 | 15 | ||||
| H2 | 0,5–1,0 | 245 | 195 | 7 | |||
| 1,0–5,0 | 245 | 195 | 7 | ||||
| 5,5–10,5 | 235 | 175 | 6 | ||||
| Without maintenance | 5,0–6,0 | 185 | 80 | 12 | |||
| 6,0–10,5 | 185 | 80 | 15 | ||||
| Without maintenance | Plates | 11,0–25,0 | 185 | 70 | 12 | ||
| 25,0–80,0 | 165 | 60 | 11 | ||||
| AMg5 | M | Sheets | 0,5–0,6 | 275 | 135 | 15 | |
| 0,6–4,5 | 275 | 145 | 15 | ||||
| 4,5–10,5 | 275 | 130 | 15 | ||||
| Without maintenance | 5,0–6,0 | 275 | 130 | 12 | |||
| 6,0–10,5 | 275 | 130 | 15 | ||||
| Plates | 11,0–25,0 | 265 | 115 | 13 | |||
| 25,0–80,0 | 255 | 105 | 12 | ||||
| AMg6 | M | Sheets | 0,5–0,6 | 305 | 145 | 15 | |
| 0,6–10,5 | 315 | 155 | 15 | ||||
| Without maintenance | 5,0–10,5 | 315 | 155 | 15 | |||
| Plates | 11,0–25,0 | 305 | 145 | 11 | |||
| 25,0–50,0 | 295 | 135 | 6 | ||||
| 50,0–80,0 | 275 | 125 | 4 | ||||
| 01570 | M | Sheets | 0,8–2,3 | 400 | 270 | 13 | |
| 2,5–4,5 | 360 | 240 | 13 | ||||
| H2 | 0,8–2,3 | 410 | 320 | 6 | |||
| N | 0,8–2,3 | 460 | 410 | 4 | |||
Guaranteed mechanical characteristics of extruded rods, pipes and profiles from Al-Mg system alloys in a state without heat treatment
| Alloy | Semi-finished products | σв, MPa | σ0.2, MPa | δ, % |
| no less | ||||
| AMg2 | Rods | 175 | – | 13 |
| Pipes | 155 | 60 | 10 | |
| AMg3 | Profiles | 175 | 75 | 12 |
| Rods | 175 | 75 | 13 | |
| Pipes | 180 | 70 | 15 | |
| AMg5 | Profiles | 255 | 115 | 15 |
| Rods | 265 | 118 | 15 | |
| Pipes | 255 | 110 | 15 | |
| AMg6 | Profiles, rods | 315 | 155 | 15 |
| Panels | 315 | 155 | 15 | |
| Pipes | 315 | 145 | 15 | |
| AMg61(1561) | Profiles | 330 | 205 | 11 |
| Rods | 330 | 155–205 | 11 | |
| Panels | 330 | 185 | 11 | |
| 01570 | Rods | 402 | 245 | 14 |
| Profiles | 392 | 255 | 14 | |
Guaranteed mechanical characteristics of forgings and stampings from Al-Mg system alloys in the annealed state depending on the direction of the fiber (D, P, V)
| Alloy | Thickness, mm | σв, MPa | σ0.2, MPa | δ, % | NV | |||||
| D | P | IN | D | P | D | P | IN | |||
| Note. Fiber direction: D - lobar; P - transverse; B - high-rise (in thickness). Indicators of stampability of sheets 2 mm thick during various shaping operations | ||||||||||
| Forgings | ||||||||||
| AMg2 | Up to 75 | 165 | 145 | 135 | – | – | 15 | 13 | 11 | 44,0 |
| AMg3 | Up to 75 | 185 | 165 | 155 | 70 | – | 15 | 12 | 10 | 44,0 |
| AMg6 | Up to 75 | 316 | 305 | 305 | 135 | 130 | 15 | 14 | 14 | 63,5 |
| 76–100 | 295 | 295 | 295 | 130 | 130 | 14 | 14 | 14 | 63,5 | |
| 100–300 | 285 | 285 | 285 | 120 | 120 | 11 | 11 | 11 | 63,5 | |
| Stampings | ||||||||||
| AMg2 | Up to 75 | 165 | 145 | 135 | – | – | 15 | 12 | 10 | 44,0 |
| AMg3 | Up to 75 | 185 | 165 | 155 | 70 | – | 15 | 12 | 10 | 44,0 |
| AMg5 | Up to 75 | 275 | – | – | 145 | – | 15 | – | – | 63,5 |
| AMg6 | Up to 75 | 315 | 305 | 305 | 155 | 130 | 15 | 14 | 14 | 63,5 |
| 76–100 | 295 | 295 | 295 | 130 | 130 | 14 | 14 | 14 | 63,5 | |
| 100–300 | 285 | 285 | 285 | 120 | 120 | 11 | 11 | 11 | 63,5 | |
Coefficients for drawing, stamping and bending radius
| Alloy and condition | Hood | Beading | Extrusion | Radius when bending at 90° | ||||
| K pr | K slave | K pr | K slave | To pl | K sf | R min, mm | R slave, mm | |
| Note. K pr and K slave - maximum and working coefficients of exhaustion; Kpl and Ksf are the coefficients of flat and spherical extrusion; R min and R slave are the minimum and working bending radii, respectively. | ||||||||
| AMg1M | 2,02 — 2,05 | — | 1,65 — 1,70 | — | 0,29 — 0,30 | 0,4 — 0,39 | (0,7 — 0,9) ∙ s | — |
| AMg2M | 2,0 — 2,6 | 1,8 — 1,85 | 1,52 — 1,56 | 1,32 — 1,40 | 0,23 — 0,26 | 0,36 — 0,42 | (0,6 — 1,0) ∙s | (1,0 — 1,5) ∙s |
| AMg3M | 1,92 | 1,86 | 1,86 | 1,63 | 0,22 — 0,25 | 0,36 — 0,32 | 1s | 2 ∙s |
| AMg4M | 1,85 — 1,90 | 1,65 — 1,70 | 1,5 — 1,65 | 1,35 — 1,45 | 0,17 — 0,19 | — | (1,0 — 1,55) ∙ s | (1,5 — 2,5) ∙ s |
| AMg5M | 1,7 — 1,87 | 1,85 — 2,02 | 1,3 — 1,5 | 1,42 — 1,62 | 0,24 — 0,29 | 0,37 — 0,46 | (0,6 — 1,0) ∙s | (2,0 — 2,5) ∙s |
| AMg6M | 2,0 — 2,06 | 1,8 — 1,85 | 1,52 — 1,56 | 1,32 — 1,40 | 0,22 — 0,25 | 0,35 — 0,40 | (0,6 — 1,0) ∙s | 2 ∙s |
| AMg6N | 1,4 | — | 1,16 | — | — | — | 5 ∙s | |
Bending radius: The radius of the cylindrical surface of the mandrel that comes into contact with the inner surface of the product when bending.
In the case of free or semi-free bends up to 180°, when a wedge or block is used, the bend radius corresponds to half the thickness of the wedge or block. (Source: “Metals and alloys. Directory.” Edited by Yu.P. Solntsev; NPO “Professional”, NPO “Peace and Family”; St. Petersburg, 2003) www.metmk.com.ua
Minerals, deposits...and native aluminum?
Aluminum reserves in nature are enormous. Among metals, it holds first place in prevalence. But the “sociability” and activity of the element have led to the fact that the metal is practically absent in its pure form.
Aluminum production in millions of tons.
There are many minerals containing aluminum:
- bauxite;
- aluminas;
- feldspars;
- nephelines;
- corundum.
So extracting aluminum raw materials is not difficult.
If all reserves on Earth are depleted (which is doubtful), then aluminum can be extracted from seawater. There its content is 0.01 mg/l.
Anyone who wants to see native aluminum will have to descend into the craters of volcanoes.
The origin of such a metal is from the very depths of our planet.
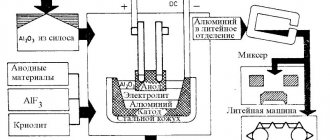
How winged metal is produced
Metal production can be divided into two stages.
- The first is the extraction of bauxite, its crushing and separation of silicon using steam.
- Second stage: alumina is mixed with molten cryolite and the mixture is exposed to electric current. During the reaction, liquid aluminum settles at the bottom of the bath.
The resulting metal is cast into ingots; then it is sent to consumers or to the production of alloys and high-purity aluminum.
The method is energy-consuming and consumes a lot of electricity.
It can be technical or ultra-clean
The resulting aluminum is called technical or unalloyed. It contains at least 99% pure metal. It is consumed by the electronics industry and is needed in the production of heat exchange and heating devices and lighting equipment.
Some of this metal is sent for additional purification, “refining.” As a result, we have a high-purity metal with an aluminum content of at least 99.995%.
It is used in electronics and in the production of semiconductors. Cable production and chemical engineering now cannot do without ultra-pure aluminum.
Interesting: before the discovery of an industrial method for producing aluminum, it was rare and cost more than gold. Our great chemist, D.I. Mendeleev, the British honored him with a gift. These were analytical balances (an indispensable thing for a chemist), whose cups were made of gold and aluminum.
Metal for wings
Without a metal like aluminum, conquering the sky is impossible. People are not given wings, but man has wanted to fly since ancient times. It is not in vain that the myth of Icarus has lived since ancient times. Attempts to take off were made several times.
But the breakthrough came in 1903, when the Wright brothers, romantics of the sky and wonderful mechanics, took an airplane into the air. This plane opened the way to the sky.
Application
Widely used as a construction material. The main advantages of aluminum in this quality are lightness, malleability for stamping, corrosion resistance (in air, aluminum is instantly covered with a durable Al2O3 film, which prevents its further oxidation), high thermal conductivity, and non-toxicity of its compounds. In particular, these properties have made aluminum extremely popular in the production of cookware, aluminum foil in the food industry and for packaging. The first three properties have made aluminum the main raw material in the aviation and aerospace industries (recently it has been slowly replaced by composite materials, primarily carbon fiber).
The main disadvantage of aluminum as a structural material is its low strength, so to strengthen it it is usually alloyed with a small amount of copper and magnesium (the alloy is called duralumin).
The electrical conductivity of aluminum is only 1.7 times less than that of copper, while aluminum is approximately 4 times cheaper per kilogram, but due to its 3.3 times lower density, to obtain equal resistance it needs approximately 2 times less weight . Therefore, it is widely used in electrical engineering for the manufacture of wires, their shielding, and even in microelectronics when depositing conductors on the surface of microcircuit crystals. The lower electrical conductivity of aluminum (3.7·107 S/m) compared to copper (5.84·107 S/m), in order to maintain the same electrical resistance, is compensated by increasing the cross-sectional area of the aluminum conductors. The disadvantage of aluminum as an electrical material is the formation of a strong dielectric oxide film on its surface, which makes soldering difficult and, due to the deterioration of contact resistance, causes increased heating at the electrical connections, which, in turn, negatively affects the reliability of the electrical contact and the condition of the insulation. Therefore, in particular, the 7th edition of the Electrical Installation Rules, adopted in 2002, prohibits the use of aluminum conductors with a cross-section of less than 16 mm².
- Due to its complex of properties, it is widely used in heating equipment.
- Aluminum and its alloys do not become brittle at ultra-low temperatures. Due to this, it is widely used in cryogenic technology. However, there is a known case of cryogenic pipes made of aluminum alloy becoming brittle due to their bending on copper cores during the development of the Energia launch vehicle.
- The high reflectivity, combined with the low cost and ease of vacuum deposition, makes aluminum the optimal material for making mirrors.
- In the production of building materials as a gas-forming agent.
- Aluminizing imparts corrosion and scale resistance to steel and other alloys, for example, valves of piston internal combustion engines, turbine blades, oil platforms, heat exchange equipment, and also replaces galvanizing.
- Aluminum sulfide is used to produce hydrogen sulfide.
- Research is underway to develop foamed aluminum as an especially strong and lightweight material.
As a reducing agent
- As a component of thermite, mixtures for aluminothermy.
- In pyrotechnics.
- Aluminum is used to recover rare metals from their oxides or halides.
- Limited use as a protector for anodic protection.
Aluminum alloys
The structural material usually used is not pure aluminum, but various alloys based on it. The designation of alloy series in this article is given for the USA (H35.1 ANSI standard) and in accordance with Russian GOST. In Russia, the main standards are GOST 1583 “Cast aluminum alloys. Technical conditions" and GOST 4784 "Aluminium and deformable aluminum alloys. Stamps." There is also UNS marking and an international standard for aluminum alloys and their marking ISO R209 b.
Aluminum profile.
- Aluminum-magnesium Al-Mg (ANSI: series 5xxx for wrought alloys and 5xx.x for alloys for shaped castings; GOST: AMg). Alloys of the Al-Mg system are characterized by a combination of satisfactory strength, good ductility, very good weldability and corrosion resistance. In addition, these alloys are characterized by high vibration resistance.
In alloys of this system containing up to 6% Mg, a eutectic system of Al3Mg2 compound with an aluminum-based solid solution is formed. The most widely used in industry are alloys containing magnesium from 1 to 5%.
An increase in the Mg content in the alloy significantly increases its strength. Each percentage of magnesium increases the tensile strength of the alloy by 30 MPa, and the yield strength by 20 MPa. In this case, the relative elongation decreases slightly and is in the range of 30-35%.
Alloys with a magnesium content of up to 3% (by weight) are structurally stable at room and elevated temperatures, even in a significantly hardened state. With increasing concentration of magnesium in the cold-worked state, the structure of the alloy becomes unstable. In addition, an increase in magnesium content above 6% leads to a deterioration in the corrosion resistance of the alloy.
To improve the strength characteristics, Al-Mg system alloys are alloyed with chromium, manganese, titanium, silicon or vanadium. They try to avoid the inclusion of copper and iron in the alloys of this system, since they reduce their corrosion resistance and weldability.
- Aluminum-manganese Al-Mn (ANSI: series 3xxx; GOST: AMts). Alloys of this system have good strength, ductility and manufacturability, high corrosion resistance and good weldability.
The main impurities in Al-Mn system alloys are iron and silicon. Both of these elements reduce the solubility of manganese in aluminum. To obtain a fine-grained structure, the alloys of this system are alloyed with titanium.
The presence of a sufficient amount of manganese ensures the stability of the structure of the cold-worked metal at room and elevated temperatures.
- Aluminum-copper Al-Cu (Al-Cu-Mg) (ANSI: series 2xxx, 2xx.x; GOST: AM). The mechanical properties of alloys of this system in a heat-strengthened state reach, and sometimes exceed, the mechanical properties of low-carbon steels. These alloys are high-tech. However, they also have a significant drawback - low corrosion resistance, which leads to the need to use protective coatings.
Manganese, silicon, iron and magnesium can be used as alloying additives. Moreover, the latter has the strongest effect on the properties of the alloy: alloying with magnesium significantly increases the strength and yield limits. The addition of silicon to the alloy increases its ability to undergo artificial aging. Alloying with iron and nickel increases the heat resistance of alloys of the second series.
Cold-hardening of these alloys after quenching accelerates artificial aging and also increases strength and stress corrosion resistance.
- Al-Zn-Mg (Al-Zn-Mg-Cu) system alloys (ANSI: 7xxx, 7xx.x series). Alloys of this system are valued for their very high strength and good manufacturability. The representative of the system - alloy 7075 is the strongest of all aluminum alloys. The effect of such high hardening is achieved due to the high solubility of zinc (70%) and magnesium (17.4%) at elevated temperatures, which sharply decreases upon cooling.
However, a significant disadvantage of these alloys is their extremely low stress corrosion resistance. The corrosion resistance of stress alloys can be increased by alloying with copper.
It is impossible not to note a pattern discovered in the 1960s: the presence of lithium in alloys slows down natural aging and accelerates artificial aging. In addition, the presence of lithium reduces the specific gravity of the alloy and significantly increases its elastic modulus. As a result of this discovery, new alloy systems Al-Mg-Li, Al-Cu-Li and Al-Mg-Cu-Li were developed.
- Aluminum-silicon alloys (silumins) are best suited for casting. Cases of various mechanisms are often cast from them.
- Complex alloys based on aluminum: avial.
Aluminum as an additive to other alloys
Aluminum is an important component of many alloys. For example, in aluminum bronzes the main components are copper and aluminum. In magnesium alloys, aluminum is most often used as an additive. For the manufacture of spirals in electric heating devices, fechral (Fe, Cr, Al) is used (along with other alloys). The addition of aluminum to the so-called “free-cut steels” facilitates their processing, giving a clear breaking of the finished part from the bar at the end of the process.
Jewelry
When aluminum was very expensive, a variety of jewelry was made from it. Thus, Napoleon III ordered aluminum buttons, and in 1889 Mendeleev was presented with scales with bowls made of gold and aluminum. The fashion for jewelry made of aluminum immediately passed when new technologies for its production appeared, reducing the cost many times over. Nowadays, aluminum is sometimes used in the production of costume jewelry.
In Japan, aluminum is used in the production of traditional jewelry, replacing silver.
Cutlery
By order of Napoleon III, aluminum cutlery was made, which was served at ceremonial dinners for him and the most honored guests. Other guests used gold and silver utensils.
Then cutlery made of aluminum became widespread; over time, the use of aluminum kitchen utensils decreased significantly, but even now they can still be seen only in some catering establishments - despite the statements of some experts about the harmfulness of aluminum to human health. In addition, such devices over time lose their attractive appearance due to scratches and their shape due to the softness of aluminum.
Utensils for the army are made from aluminum: spoons, pots, flasks.
Glass making
Fluoride, phosphate and aluminum oxide are used in glass making.
Food industry
Aluminum is registered as a food additive E173.
Aluminum gel is a gelatinous precipitate formed during the rapid precipitation of aluminum hydroxide from saline solutions, does not have a crystalline structure and contains a large amount of water, is used as a basis for antacids, analgesics and enveloping agents (algeldrate; mixed with magnesium hydroxide - almagel, maalox, gastracid and etc.) for diseases of the gastrointestinal tract.
Military industry
The cheapness and weight of the metal led to its widespread use in the production of small arms, in particular machine guns and pistols.
Aluminum and its compounds in rocket technology
Aluminum and its compounds are used as a highly efficient propellant in two-propellant rocket propellants and as a combustible component in solid rocket propellants. The following aluminum compounds are of greatest practical interest as rocket fuel:
- Powdered aluminum as fuel in solid rocket propellants. It is also used in the form of powder and suspensions in hydrocarbons.
- Aluminum hydride.
- Aluminum boranate.
- Trimethylaluminum.
- Triethylaluminum.
- Tripropylaluminum.
Triethylaluminum (usually mixed with triethylboron) is also used for chemical ignition (as a starting fuel) in rocket engines, since it spontaneously ignites in oxygen gas. Rocket fuels based on aluminum hydride, depending on the oxidizer, have the following characteristics:
| Oxidizer | Specific thrust (P1, s) | Combustion temperature, °C | Fuel density, g/cm³ | Speed increase, Δ V id, 25, m/s | Weight content of fuel, % |
| Fluorine | 348,4 | 5009 | 1,504 | 5328 | 25 |
| Tetrafluorohydrazine | 327,4 | 4758 | 1,193 | 4434 | 19 |
| ClF3 | 287,7 | 4402 | 1,764 | 4762 | 20 |
| ClF5 | 303,7 | 4604 | 1,691 | 4922 | 20 |
| Perchloryl fluoride | 293,7 | 3788 | 1,589 | 4617 | 47 |
| Oxygen fluoride | 326,5 | 4067 | 1,511 | 5004 | 38,5 |
| Oxygen | 310,8 | 4028 | 1,312 | 4428 | 56 |
| Hydrogen peroxide | 318,4 | 3561 | 1,466 | 4806 | 52 |
| N2O4 | 300,5 | 3906 | 1,467 | 4537 | 47 |
| Nitric acid | 301,3 | 3720 | 1,496 | 4595 | 49 |
Aluminum energy uses aluminum as a universal secondary energy carrier. Its uses in this capacity:
- Oxidation of aluminum in water to produce hydrogen and thermal energy.
- Oxidation of aluminum with air oxygen to produce electricity in air-aluminum electrochemical generators.
Mechanical properties of aluminum
What are mechanical properties?
The mechanical properties of aluminum, like other materials, are properties that are associated with the elastic and inelastic response of the material to the application of a load to it, including the relationship between stress and strain. Examples of mechanical properties are:
- modulus of elasticity (tensile, compressive, shear)
- tensile strength (tensile, compressive, shear)
- yield strength
- fatigue limit
- elongation (relative) at break
- hardness.
Mechanical properties are often mistakenly referred to as physical properties.
The mechanical properties of materials, including aluminum and its alloys, that are obtained by tensile testing of the material, such as tensile modulus, tensile strength, tensile yield strength and elongation, are called tensile mechanical properties.
Elastic modulus
The modulus of elasticity, often called Young's modulus, is the ratio of the stress that is applied to a material to the corresponding strain in the range where they are directly proportional to each other.
There are three types of stresses and, accordingly, three types of elastic moduli for any material, including aluminum:
- tensile modulus of elasticity
- compressive modulus of elasticity
- shear modulus of elasticity (shear modulus of elasticity).
Table - Tensile elastic moduli of aluminum and other metals [1]
Tensile strength
The ratio of the maximum load before failure of a sample when testing it in tension to the original cross-sectional area of the sample. The terms “tensile strength” and “tensile strength” are also used.
Yield strength
The stress required to achieve a specified small plastic deformation in aluminum or other material under uniaxial tensile or compressive load.
If the plastic deformation under tensile load is specified as 0.2%, then the term “yield strength 0.2%” (Rp0.2) is used.
Figure - Typical stress-strain diagram for aluminum alloys
Elongation (at break)
Often called "relative elongation". An increase in the distance between two marks on a test specimen that occurs as a result of the specimen deforming under tension until it breaks between the marks.
The amount of elongation depends on the cross-sectional dimensions of the sample. For example, the amount of elongation that is obtained when testing an aluminum sheet specimen will be lower for a thin sheet than for a thick sheet. The same applies to extruded aluminum profiles.
Extension A
Elongation in percent after sample rupture at an initial distance between marks of 5.65 √ S
0, where S0 is the initial cross-sectional area of the test sample. The outdated designation of this quantity A5 is currently not used. A similar value in Russian-language documents is designated δ5.
It is easy to check that for round samples this distance between the original marks is calculated as 5·d.
Extension A50mm
The percentage elongation after specimen rupture, relative to the original length between the 50 mm marks and the constant original width of the test specimen (typically 12.5 mm). In the USA, a distance between marks of 2 inches is used, that is, 50.8 mm.
Shear strength
The maximum specific stress, that is, the maximum load divided by the original cross-sectional area that a material can withstand when tested in shear. Shear strength is typically 60% of tensile strength.
Shear strength is an important quality characteristic of rivets, including aluminum ones.
Poisson's ratio
The relationship between longitudinal elongation and transverse shortening of a section in a uniaxial test. For aluminum and all aluminum alloys in all states, Poisson's ratio is typically 0.33 [2].
Hardness
The resistance of a metal to plastic deformation, usually measured by making an impression.
Brinell Hardness (HB)
Penetration resistance of a spherical indenter under standardized conditions.
For aluminum and aluminum alloys, the hardness of NV is approximately equal to 0.3 Rm, where Rm is the tensile strength expressed in MPa [2].
If a tungsten carbide indenter is used, the designation HBW is used.
Vickers Hardness (HV)
Penetration resistance of a square pyramid diamond indenter under standardized conditions. Hardness HV is approximately equal to 1.10·HB [2].
Fatigue
The tendency of a metal to fail under prolonged cyclic stress that is well below its tensile strength.
Fatigue strength
The maximum stress amplitude that a product can withstand for a given number of loading cycles. Typically expressed as the stress amplitude that gives a 50% probability of failure after a given number of loading cycles [2].
Fatigue endurance
The limiting stress below which a material will withstand a specified number of stress cycles [2].
Mechanical properties of aluminum and aluminum alloys
The tables below [3] present typical mechanical properties of aluminum and aluminum alloys:
- tensile strength
- tensile yield strength
- tensile elongation
- fatigue endurance
- hardness
- elastic modulus
Mechanical properties are presented separately:
- for aluminum alloys hardened by work hardening.
- for aluminum alloys, hardened by heat treatment.
These mechanical properties are typical. This means that they are only suitable for comparative purposes and not for engineering calculations. In most cases, they are average values for various product sizes, shapes and manufacturing methods.
Source:
- Materials of the German Aluminum Association
- Global Advisory Group GAG – Guidance “Terms and Definitions” – 2011-01
- Aluminum and Aluminum Alloys. - ASM International, 1993.
aluminum-guide.ru
Toxicity
It has a slight toxic effect, but many water-soluble inorganic aluminum compounds remain in a dissolved state for a long time and can have a harmful effect on humans and warm-blooded animals through drinking water. The most toxic are chlorides, nitrates, acetates, sulfates, etc. For humans, the following doses of aluminum compounds (mg/kg body weight) have a toxic effect when ingested: aluminum acetate - 0.2-0.4; aluminum hydroxide - 3.7-7.3; aluminum alum - 2.9. Primarily affects the nervous system (accumulates in nervous tissue, leading to severe disorders of the central nervous system). However, the neurotoxicity of aluminum has been studied since the mid-1960s, since the accumulation of the metal in the human body is prevented by its elimination mechanism. Under normal conditions, up to 15 mg of the element per day can be excreted in the urine. Accordingly, the greatest negative effect is observed in people with impaired renal excretory function.
The standard for aluminum content in drinking water is 0.2 mg/l. In this case, this MPC can be increased to 0.5 mg/l by the chief state sanitary doctor for the relevant territory for a specific water supply system.
Sources
- https://www.RusCable.ru/info/general/aluminum/
- https://chemege.ru/aluminium/
- https://TheMineral.ru/metally/alyuminij
- https://chem.ru/aljuminij.html
- https://himsnab-spb.ru/article/ps/al/
Chemical composition and typical mechanical properties of heat-resistant aluminum alloys
| Brand | Chemical composition, % | Mechanical properties | |||||||
| Al | Cu | Mg | Mn | Si | other elements | in , MPa | 0.2 , M p a | , % | |
| AK4-1 | the basis | 1,9-2,5 | 1,4-1,8 | — | 0,35 | 0.8-1.4 Fe 0.8-1.4 Ni 0.02-0.1Ti | 430 | 280 | 13 |
| D 20 | the basis | 6-7 | — | 0,4-0,8 | — | 0.1-0.2Ti | 400 | 250 | 12 |
The strengthening phases of heat-resistant alloys are CuAl2, Al2CuMg, Al9FeNi and Al6CuNi. After quenching and aging with partial decomposition of the solid solution, these phases are released in the form of dispersed particles, which significantly increase the heat resistance of the alloys. Table 4 presents the chemical composition and mechanical properties after heat treatment of the most commonly used alloys.
The high heat resistance of the D20 alloy is achieved due to the high content of copper and manganese with titanium
Alloys for forging and stamping. These aluminum alloys have high ductility and satisfactory casting properties. The main alloying elements are copper, magnesium, manganese and silicon. Forging and stamping of alloys is carried out at a temperature of 450°C. To increase strength, heat treatment is carried out, consisting of hardening and artificial aging. Strengthening phases during aging are Mg2Si, CuAl2, AlxMg5CuSi4. Table 5 presents the chemical composition and main mechanical properties of the alloys.
Table 5
Chemical composition and mechanical properties of aluminum alloys for forging and stamping
| Brand | Element content, % | Mechanical properties | |||||
| Cu | Mg | Mn | Si | in , MPa | 0,2, MPa | , % | |
| AK 6 | 1,8-2,6 | 0,4-0,8 | 0,4-0,8 | 0,7-1,2 | 420 | 300 | 12 |
| AK 8 | 3,9-4,8 | 0,4-0,8 | 0,4-1,0 | 0,6-1,2 | 480 | 380 | 10 |
These alloys are easy to cut and can be welded satisfactorily by resistance and argon arc welding. Casting properties are improved by adding silicon. However, these alloys are prone to intergranular and stress corrosion. They are used for the manufacture of sub-engine frames, fasteners, helicopter rotor blades, etc.
Cast aluminum alloys
Casting alloys must have high fluidity, relatively low shrinkage, low tendency to form hot cracks and pores, good mechanical properties and corrosion resistance.
Alloys that have eutectic in their structure have the best casting properties. The formation of eutectic depends on the concentration of alloying elements, i.e. their content must be greater than the maximum solubility in aluminum.
Aluminum-silicon, aluminum-copper, aluminum-magnesium alloys are used as casting alloys.
These alloys are marked with the letters AL followed by a number. The letter “A” means that it is an aluminum alloy, the letter “L” is a cast alloy, and the number corresponds to the serial number from GOST, for example, AL2, AL4, etc.
Silumins
. Al-Si alloys, called silumins, are widely used. Their composition is close to a eutectic alloy (Appendix 3), so they have high casting properties. The chemical composition and properties of some silumins are presented in Table 6.
The most widely used alloy is AL2, which contains eutectic (), -phase – silicon crystals in its structure. When the eutectic solidifies, silicon is released in the form of large needle-shaped crystals, which seem to cut into the plastic α-solid solution. An alloy with such a structure has poor mechanical properties.
Table 6
studfiles.net