Synonyms: Thin mixtures of native copper and cuprite were called cuprocuprite (Vernadsky, 1910). Whitneyite—whitneyite (Ghent, 1859) and darwinite (Forbes, 1860)—arsenic copper that forms mixtures with algodonite.
Group
origin of name
The Latin name for copper, cuprum, comes from the name of the island of Cyprus, from where copper was imported in ancient times. The origin of the Russian name is unclear.
The English name for the mineral Copper is Copper.
Copper nugget
Chemical composition
Sometimes it contains impurities of Fe, Ag, Pb, Au, Hg, Bi, Sb, V, Ge3 (silver copper with 3-4% Ag, ferruginous copper - 2.5% Fe and golden copper - 2-3% Au). Impurities are observed more often in primary native copper; Recycled copper is usually purer. Composition of native copper from the Shamlug deposit (Armenia): Cu - 97.20 -97.46%, Fe - 0.25%; 98.3% Cu or more was determined in copper from Altai deposits.
Crystallographic characteristics
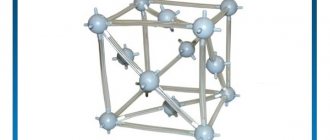
Syngony. Cubic.
Class. Hexoctahedral.
Crystal structure
The crystal structure is characterized by a face-centered lattice; Copper atoms are located at the corners and in the centers of the faces of the elementary cube. This is a formal expression of the fact that in the structure of copper there is a close packing (the so-called cubic close packing) of metal atoms with a radius of 1.27 A and a distance between nearest atoms of 2.54 A with a space fulfillment of 74.05%. Each Cu atom is surrounded by 12 similar ones (coordination number 12), located around it at the vertices of the so-called Archimedean cuboctahedron.
Main forms : a (100), d (110), o (111), l (530), e (210), h (410).
Areas of use
Copper is in greatest demand in electrical engineering. Due to its low electrical resistance, it is actively used for the manufacture of wires, cables, conductive elements of transformers, and electric motor windings are made from it. More than half of all mined copper is consumed by the industry.
The metal has good strength and ductility, which makes it suitable for the manufacture of products of complex shapes: household items, jewelry; for creating seamless pipes. Copper pipes are often used in equipment and various engineering systems to organize heat exchange. The properties of copper such as low coefficient of thermal expansion and high thermal conductivity are used here.
Copper alloys are widely used:
- bronze - a compound with tin, the name is also used for alloys of copper with other metals, such as aluminum, lead, silicon, beryllium and others;
- brass - with zinc;
- cupronickel, nickel silver, manganin - with nickel;
Copper is also included as one of the components in other compounds, for example, in duralumin. Jewelers often use copper alloys with the addition of gold.
Form of being in nature
The appearance of crystals . The shape of the crystals is cubic, tetrahexahedral, dodecahedral, less often octahedral (possibly pseudomorphs of cuprite). The edges are often rough, with depressions or elevations. Simple crystals are rare.
Doubles.
Intergrowth twins along (111) are common, sometimes polysynthetic, often lamellar in the direction of the twin axis or elongated parallel to the diagonal twins of the plane. Typically, crystals (simple and twins) are unevenly developed: elongated, shortened or deformed. Characteristic are dendritic forms, which represent uniform accretion of many crystals (uniformly deformed or regular) in any one direction. These are, for example, twin (111) crystals, elongated along the 2nd order symmetry axis and accreted parallel to the faces of the rhombic dodecahedron) or intergrowths of regular twin crystals, branching in the direction of the edges and diagonals of octahedral faces, as well as parallel intergrowths of crystals elongated in the direction 4th order axes. In continuous deposits of native copper during etching, signs of collective crystallization are revealed with the development of large grains at the expense of smaller zonal grains of irregular shape.
Aggregates. Distorted crystals, in single irregular grains, dendrite-like intergrowths, thread-like, wire-like, mossy formations, thin plates, concretions, powdery accumulations and solid masses weighing up to several hundred tons.
Dendrites
Physical properties
Optical
The color of a fresh fracture is light pink, quickly turning into copper-red, then brown; often with yellow or mottled tarnish.
The streak is copper-red, shiny.
Metallic shine.
Low tide
Transparency. Opaque. In the thinnest plates it shines through in green.
Refractive indices
Ng = , Nm = and Np =
Mechanical
Hardness 2.5-3.
Density 8.4—8.9
No cleavage is observed.
The fracture is splintered and hooked.
Chemical properties
It dissolves easily in dilute HNO3 and aqua regia, in H2SO4 when heated, and with difficulty in HCl. Ammonia dissolves in an aqueous solution, turning it blue. In polished sections it is etched with all the main reagents. The internal structure is easily revealed using NH4OH + H2O2 or HCl + CrO3 (50% solution).
Other properties
Very malleable and viscous. Electrical conductivity is very high; is significantly reduced by impurities.
Heating behavior. Pure copper melts at 1083°. Thermal conductivity is slightly less than that of silver.
Extraction methods
Depending on the depth of the ore, an open or closed mining method is chosen. With the closed mining method, deep mines are built. Elevators are built to move workers, equipment and mined rock. The rock is chipped and crushed. Then they are collected using machines equipped with buckets, and using special technical means - trolleys, elevators - they are sent to the processing site.
If the ore lies at a depth of up to half a kilometer, then it is mined using an open pit method. A lot of preparatory work is required. Before mining begins, layers of waste rock are removed to gain access to the deposit. Here, work is easier to automate, it is easier to deliver people and equipment, and the costs of developing infrastructure are lower.
Artificial production of the mineral.
It can be easily obtained from melts or by electrolysis from solutions of copper salts.
Diagnostic signs
Similar minerals
Recognized by the red color of the fresh surface, shiny streak, medium hardness and malleability, usually covered with greenish, black, blue deposits of oxidized copper minerals. Under a microscope in reflected light, it is easily determined by color and reflectivity.
Associated minerals. Cuprous gold, chalcocite, calcite, diopside, apatite, sphene, magnetite, malachite, barite, quartz, chalcopyrite.
Copper minerals and their formulas
Synonyms: Thin mixtures of native copper and cuprite were called cuprocuprite (Vernadsky, 1910).
Whitneyite—whitneyite (Ghent, 1859) and darwinite (Forbes, 1860)—arsenic copper that forms mixtures with algodonite. The Latin name for copper, cuprum, comes from the name of the island of Cyprus, from where copper was imported in ancient times. The origin of the Russian name is unclear.
The English name for the mineral Copper is Copper.
Copper nugget
- Chemical composition
- Varieties
- Crystallographic characteristics
- Form of being in nature
- Physical properties
- Chemical properties. Other properties
- Diagnostic signs. Satellites.
- Origin of the mineral
- Place of Birth
- Practical use
- Physical research methods
- Crystal optical properties in thin preparations (sections)
- Buy
Origin and location
Hydrothermal. Accumulates in placers. Nuggets weighing up to 450 tons are described as unique phenomena.
Native copper is formed under reducing conditions during various geological processes; a significant part of it is released from hydrothermal solutions. Occurs as microscopic deposits in many, predominantly mafic, igneous rocks exposed to hydrothermal solutions, such as serpentinized peridotites, dunites and serpentinites. In this case, the appearance of native copper may be associated with the decomposition of previously formed copper sulfides, for example, cubanite (Urals, Transcaucasia). A similar origin can be attributed to native copper in amphibolitized basic rocks of the Serov region of the Sverdlovsk region. In the Karabash copper gold deposit of the Chelyabinsk region, native copper is observed in vein-like bodies of diopside-garnet rocks occurring among serpentinites; native copper here is characterized by an association with cuprous gold, chalcocite, calcite, diopside, apatite, sphene, magnetite, etc. In some ancient volcanic rocks (melafyres, diabases, etc.), metamorphosed under the influence of vapors, gases and hydrothermal solutions, copper performs tonsils, forms cement between the minerals of altered lava, fills voids and cracks; accompanied by hydrothermal minerals: analcime, laumontite, prehnite, datolite, adularia, chlorite, epidote, pumpelyite, quartz, calcite. The largest deposits of this type are located on the Keweenaw Peninsula in the Lake Superior region (Michigan, USA), where mineralization is confined to the Upper Proterozoic strata. The bulk of copper is mined from melafires and conglomerates, but the largest copper deposits (up to 400 tons or more) are found in calcite veins containing native silver and domeicite.
Copper nugget
What is copper ore
Copper ore, like any other, is a conglomerate of substances, rocks, minerals, the content of the desired substance in which is so high that it is considered appropriate for mining. It is worth saying that along with the so-called Cuprum (the Latin name for copper), other useful elements are also mined in its ore in even smaller proportions. Copper itself begins to be mined in ores in which its amount exceeds 0.5%.
Yes, in its pure form, copper is found in nature even more often than aluminum, but still this figure is approximately one percent of the global reserves, because mining is still carried out from ores. The following groups of ores are distinguished by places of formation and composition: carbonate, sulfide, copper-nickel, porphyry copper (hydrothermal), skarn, stratiform.
Place of Birth
Segregations of native copper were observed in the diabases of Novaya Zemlya, in the traps of the Siberian Platform, among the main effusive rocks in Italy, on the Faroe Islands (Denmark), in Nova Scotia (Canada) and in other places. Representatives of rare types of hypogene deposits of native copper are the Franklin zinc-manganese deposit (New Jersey, USA) and the Longbahn and Jacobsberg manganese deposits (Sweden). Apparently, native copper deposits weighing up to several tons from the previously developed Kalmaktas deposit in Kazakhstan, represented in museums by excellent examples, are hypogenic. In the oxidation zone, especially in its lower parts, native copper is mainly an early alteration product of sulfide copper minerals, mainly chalcocite. It consists mainly of irregularly shaped deposits, less often - crystals and dendrite-like aggregates. Most often, native copper is accompanied by chalcocite, cuprite, calcite, and limonite. It is observed in a number of deposits in Kazakhstan (Dzhezkazgan, Berkara, Uspenskoye, etc.), Rudny Altai (Belousovskoye, Zyryanovskoye, Chudak, Talovskoye, etc.), the USA (Bisbee and Clifton-Morenci in Arizona, Tintik in Utah, etc.) . Part of the native copper in the oxidation zone arises by deposition from solutions containing copper sulfate. This is, for example, native copper, which forms segregations in cavities among limonite aggregates, sometimes in association with cuprite (Mednorudyanekoe deposit in the Sverdlovsk region, etc.). Pseudomorphoses of native copper are known, formed in the oxidation zone of chalcocite, cuprite, antlerite, chalcanthite, azurite, calcite, aragonite and other minerals. Particularly beautiful samples of native copper (crystals and dendritic intergrowths) come from the Turinsky mines of the Sverdlovsk region. In some mine workings, so-called cement copper is released from copper-containing waters on iron objects in the form of films and crusts. There are also cases of copper formation on half-rotten remains of fastening wood. In increased quantities, native copper is observed in some sedimentary rocks (sandstones, clays, marls) containing plant remains, in the form of irregularly shaped secretions, sometimes in pseudomorphs on wood or in the form of nodules. These are, for example, the Permian copper sandstones of certain regions of Russia (Ural region, Tatarstan, etc.), the Naukat sandstones in Kyrgyzstan, and the Cretaceous copper sandstones of Korokoro and Kobritsos in Bolivia, etc. The formation of native copper in some peatlands, for example, in the Sverdlovsk region, is also associated with reduction processes. region - along the Levikha River in the Tagila River basin and in the Sysert region. In the form of pebbles and grains, native copper is found in Russia in some placers: in the Urals, along the Yenisei, along the Bolshaya Sarkhoi River in Buryatia, along the Chorokh River in Georgia, on the Commander Islands and other places. In the state of Connecticut (USA), native copper was found in glacial sediments in the form of segregations weighing up to 75 kg. Small, irregularly shaped deposits of native copper were noted in the native iron of the Vengerovo meteorite in association with troilite.
Copper
Crystal optical properties in thin preparations (sections)
In polished sections, pink in reflected light. Reflectivity (in%): for green rays - 61, for orange - 83, for red - 89. Isotropic. Refractive indices (according to Kundt) in prisms for red light - 0.45, for white - 0.65, for blue - 0.95; in reflective light (according to Drude) for Na-light 0.641, for red - 0.580. The absorption coefficient for Na-light is 4.09, for red light is 5.24.
Copper. Branched dendrites. Russia
Copper. Branched dendrites. Russia
cougar
Copper. cougar