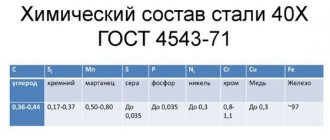
Copper is a ductile golden-pink metal with a characteristic metallic luster.
In the periodic system of D.I. Mendeleev, this chemical element is designated as Cu (Cuprum) and is located under serial number 29 in group I (side subgroup), in the 4th period. The Latin name Cuprum comes from the name of the island of Cyprus. There are known facts that in Cyprus back in the 3rd century BC there were copper mines and local craftsmen smelted copper. You can buy copper from the KUPRUM company.
According to historians, society has been familiar with copper for about nine thousand years. The most ancient copper products were found during archaeological excavations in the area of modern Turkey. Archaeologists have discovered small copper beads and plates used to decorate clothing. The finds date back to the turn of the 8th-7th millennium BC. In ancient times, copper was used to make jewelry, expensive dishes, and various tools with thin blades.
A great achievement of ancient metallurgists can be called the production of an alloy with a copper base - bronze.
Basic properties of copper
Physical properties.
In air, copper acquires a bright yellowish-red hue due to the formation of an oxide film. Thin plates have a greenish-blue color when examined through them. In its pure form, copper is quite soft, malleable and easily rolled and drawn. Impurities can increase its hardness.
The high electrical conductivity of copper can be called the main property that determines its predominant use. Copper also has very high thermal conductivity. Impurities such as iron, phosphorus, tin, antimony and arsenic affect the basic properties and reduce electrical and thermal conductivity. According to these indicators, copper is second only to silver.
Copper has high densities, melting points and boiling points. An important property is also good resistance to corrosion. For example, at high humidity, iron oxidizes much faster.
Copper lends itself well to processing: it is rolled into copper sheets and copper rods, and drawn into copper wire with a thickness brought to thousandths of a millimeter. This metal is diamagnetic, that is, it is magnetized against the direction of the external magnetic field.
Chemical properties.
Copper is a relatively low-active metal. Under normal conditions in dry air, its oxidation does not occur. It reacts easily with halogens, selenium and sulfur. Acids without oxidizing properties have no effect on copper. There are no chemical reactions with hydrogen, carbon and nitrogen. In humid air, oxidation occurs to form copper (II) carbonate, the top layer of platinum. Copper is amphoteric, meaning it forms cations and anions in the earth's crust. Depending on the conditions, copper compounds exhibit acidic or basic properties.
What is solubility?
This is the process of formation of homogeneous systems in the form of solutions when one compound interacts with other substances. Their components are individual molecules, atoms, ions and other particles. The degree of solubility is determined by the concentration of the substance that was dissolved when obtaining a saturated solution.
The unit of measurement is most often percentages, volume fractions or weight fractions. The solubility of copper in water, like other solid compounds, is subject only to changes in temperature conditions. This dependence is expressed using curves. If the indicator is very small, then the substance is considered insoluble.
Methods for obtaining copper
In nature, copper exists in compounds and in the form of nuggets. The compounds are represented by oxides, bicarbonates, sulfur and carbon dioxide complexes, as well as sulfide ores. The most common ores are copper pyrite and copper luster. The copper content in them is 1-2%. 90% of primary copper is mined using the pyrometallurgical method and 10% using the hydrometallurgical method.
1. The pyrometallurgical method includes the following processes: enrichment and roasting, smelting for matte, purging in a converter, electrolytic refining. Copper ores are enriched by flotation and oxidative roasting. The essence of the flotation method is as follows: copper particles suspended in an aqueous medium adhere to the surface of air bubbles and rise to the surface. The method allows you to obtain copper powder concentrate, which contains 10-35% copper.
Copper ores and concentrates with a significant sulfur content are subject to oxidative roasting. When heated in the presence of oxygen, sulfides are oxidized, and the amount of sulfur is reduced by almost half. Poor concentrates containing 8-25% copper are roasted. Rich concentrates containing 25-35% copper are melted without resorting to roasting.
The next stage of the pyrometallurgical method for producing copper is smelting for matte. If lump copper ore with a large amount of sulfur is used as a raw material, then smelting is carried out in shaft furnaces. And for powdered flotation concentrate, reverberatory furnaces are used. Melting occurs at a temperature of 1450 °C.
In horizontal converters with side blowing, the copper matte is blown with compressed air in order for the oxidation of sulfides and ferrum to occur. Next, the resulting oxides are converted into slag, and sulfur into oxide. The converter produces blister copper, which contains 98.4-99.4% copper, iron, sulfur, as well as small amounts of nickel, tin, silver and gold.
Blister copper is subject to fire and then electrolytic refining. Impurities are removed with gases and converted into slag. As a result of fire refining, copper is formed with a purity of up to 99.5%. And after electrolytic refining, the purity is 99.95%.
2. The hydrometallurgical method involves leaching copper with a weak solution of sulfuric acid, and then separating copper metal directly from the solution. This method is used for processing low-grade ores and does not allow for the associated extraction of precious metals along with copper.
Usually solutions of copper salts are colored blue (or blue), why is our solution yellow-brown? The fact is that the blue color is due to the presence of hydrated [Cu(H2O)n]2+ cations. Dilute solutions of copper chloride CuCl2 are blue. But in strong solutions of hydrochloric acid, copper (II) chloride forms a brown complex:
CuCl2 + 2HCl <= > H2[CuCl4]
In other words, chloride ions displace water molecules from the inner coordination sphere. If a solution of H2[CuCl4] is diluted with water, the reverse process will occur - water molecules will return to the inner coordination sphere of copper cations, and the solution will first turn green and then blue.
In the absence of hydrochloric acid, a complex [CuCl4]2- is also formed in strong solutions of CuCl2, and copper ions are located in its outer coordination sphere:
2CuCl2 <=> Cu[CuCl4]
Many transition metals behave similarly: iron, cobalt, nickel, zinc, silver, gold, and platinum metals can form chloride complexes. Remember that when gold is dissolved in aqua regia, chlorauric acid H[AuCl4] is formed.
What conclusions can be drawn? In the presence of air, copper dissolves in hydrochloric acid to form copper (II) chloride, but hydrogen is not released. In the absence of air, the reaction does not occur. It is not difficult to guess that atmospheric oxygen plays the role of an oxidizing agent:
2Cu + 4HCl + O2 = 2CuCl2 + 2H2O
Formally, this reaction can be considered as a combination of two stages:
2Cu + O2 = 2CuO
CuO + 2HCl = CuCl2 + H2O
however, the actual mechanism of this process is completely different. In addition, in strong HCl solutions, copper chloride transforms into the [CuCl4]2- complex.
Thus, copper really does not react with hydrochloric acid in the absence of oxidizing agents, but what is this statement worth in practice? After all, we are surrounded by an atmosphere, and the role of an oxidizing agent is played quite successfully by oxygen in the air - even at room temperature.
What happens if you use a stronger oxidizing agent? To answer this question, the author took the first bottle with copper wire (in which the reaction did not proceed), poured out two-thirds of the hydrochloric acid, slightly diluted the remaining acid with water and added 5 ml of 30% peroxide. The solution immediately turned yellow-green and gas began to evolve. After a few seconds, the reaction accelerated sharply, and after a minute almost all the contents splashed out - a small amount of almost black solution of copper (II) chloride remained inside the bottle:
Cu + 2HCl + H2O2 = CuCl2 + 2H2O
Realizing his mistake, the author began adding hydrogen peroxide and hydrochloric acid in small portions. As a result, all the copper wire quickly dissolved. A greenish-brown solution of CuCl2 (or rather, H2[CuCl4]) was formed. Using the above method, you can easily dissolve copper without nitric or concentrated sulfuric acid.
Copper Applications
Due to their valuable qualities, copper and copper alloys are used in the electrical and electrical engineering industries, in radio electronics and instrument making. There are alloys of copper with metals such as zinc, tin, aluminum, nickel, titanium, silver, and gold. Less commonly used are alloys with non-metals: phosphorus, sulfur, oxygen. There are two groups of copper alloys: brass (alloys with zinc) and bronze (alloys with other elements).
Copper is highly environmentally friendly, which allows its use in the construction of residential buildings. For example, a copper roof, due to its anti-corrosion properties, can last more than a hundred years without special care or painting.
Copper in alloys with gold is used in jewelry. This alloy increases the strength of the product, increases resistance to deformation and abrasion.
Copper compounds are characterized by high biological activity. In plants, copper takes part in the synthesis of chlorophyll. Therefore, it can be seen in the composition of mineral fertilizers. A lack of copper in the human body can cause deterioration in blood composition. It is found in many food products. For example, this metal is found in milk. However, it is important to remember that excess copper compounds can cause poisoning. This is why you should not cook food in copper cookware. During boiling, large amounts of copper can leach into food. If the dishes inside are covered with a layer of tin, then there is no danger of poisoning.
In medicine, copper is used as an antiseptic and astringent. It is a component of eye drops for conjunctivitis and solutions for burns.
Solubility of copper in nitric acid
This reaction is possible due to the fact that the process of oxidation of the metal with a strong reagent occurs. Nitric acid in diluted and concentrated form exhibits oxidizing properties with the dissolution of copper.
In the first option, the reaction produces copper nitrate and nitrogen divalent oxide in a ratio of 75% to 25%. The process with dilute nitric acid can be described by the following equation:
8HNO3 + 3Cu → 3Cu(NO3)2 + NO + NO + 4H2O.
In the second case, copper nitrate and nitrogen oxides are obtained, divalent and tetravalent, the ratio of which is 1 to 1. This process involves 1 mole of metal and 3 moles of concentrated nitric acid. When copper dissolves, the solution heats up strongly, resulting in thermal decomposition of the oxidizing agent and the release of an additional volume of nitrogen oxides:
4HNO3 + Cu → Cu(NO3)2 + NO2 + NO2 + 2H2O.
The reaction is used in small-scale production associated with recycling scrap or removing coatings from waste. However, this method of dissolving copper has a number of disadvantages associated with the release of large amounts of nitrogen oxides. To capture or neutralize them, special equipment is required. These processes are very expensive.
The dissolution of copper is considered complete when the production of volatile nitrogen oxides completely ceases. The reaction temperature ranges from 60 to 70 °C. The next step is to drain the solution from the chemical reactor. At its bottom there are small pieces of metal that have not reacted. Water is added to the resulting liquid and filtered.
Dissolution in ammonia
The process often occurs by passing NH3 in gaseous form over hot metal. The result is the dissolution of copper in ammonia, the release of Cu3N. This compound is called monovalent nitride.
Its salts are exposed to ammonia solution. The addition of such a reagent to copper chloride leads to the formation of a precipitate in the form of hydroxide:
CuCl2 + NH3 + NH3 + 2H2O → 2NH4Cl + Cu(OH)2↓.
Excess ammonia promotes the formation of a complex type compound that is dark blue in color:
Cu(OH)2↓+ 4NH3 → [Cu(NH3)4] (OH)2.
This process is used to determine cupric ions.
Solubility of copper in iron
As a result of this process, pseudo-alloys of Fe and Cu are formed. For metallic iron and copper, limited mutual solubility is possible. Its maximum values are observed at a temperature of 1099.85 °C. The degree of solubility of copper in the solid form of iron is 8.5%. These are small numbers. The dissolution of metallic iron in the solid form of copper is about 4.2%.
Reducing the temperature to room values makes the mutual processes insignificant. When metallic copper is melted, it is able to well wet iron in solid form. When producing Fe and Cu pseudo-alloys, special blanks are used. They are created by pressing or baking iron powder in pure or alloyed form. Such workpieces are impregnated with liquid copper, forming pseudo-alloys.
Note:
205* The empirical radius of the copper atom according to [1] and [3] is 128 pm.
206* Covalent radius of copper according to [1] and [3] is 132±4 pm and 117 pm, respectively.
401* The density of copper according to [3] is 8.92 g/cm3 (at 0 °C and other standard conditions, the state of the substance is a solid).
402* The melting point of copper according to [3] and [4] is 1083.4 °C (1356.55 K, 1982.12 °F) and 1083 °C (1356.15 K, 1981.4 °F), respectively.
403* The boiling point of copper according to [3] and [4] is 2567 °C (2840.15 K, 4652.6 °F) and 2543 °C (2816.15 K, 4609.4 °F), respectively.
407* The specific heat of fusion (enthalpy of fusion ΔHmelt) of copper according to [3] and [4] is 13.01 kJ/mol and 13 kJ/mol, respectively.
408* The specific heat of evaporation (enthalpy of boiling ΔHboiling) of copper according to [3] and [4] is 304.6 kJ/mol and 302 kJ/mol, respectively.
Solubility in sulfuric acid
Under normal conditions, this reaction does not occur. The factor determining the dissolution of copper in sulfuric acid is its strong concentration. A dilute medium cannot oxidize the metal. The dissolution of copper in concentrated sulfuric acid proceeds with the release of sulfate.
The process is expressed by the following equation:
Cu + H2SO4 + H2SO4 → CuSO4 + 2H2O + SO2.