Copper belongs to the group of non-ferrous metals most widely used in industry. The serial number of copper in the periodic system of D.I. Mendeleev is 29, atomic weight A = 63.57. Copper has a face-centered cubic lattice (fcc) with a period a = 3.607 Å.
Specific gravity of copper g = 8.94 g/cm3, melting point - 1083 0C. Pure copper has high thermal and electrical conductivity. The electrical resistivity of copper is 0.0175 μΩ×m, thermal conductivity l = 395 W/(m×deg). Ultimate strength sв = 200…250 MPa, hardness 85…115 НВ, relative elongation d = 50%, relative contraction y = 75%.
Copper is a non-magnetic metal. It has good manufacturability: it can be processed by pressure, cutting, is easy to polish, is well soldered and welded, and has high corrosion resistance. The main area of application is the electrical industry.
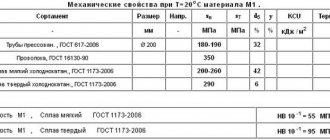
The electrical conductivity of copper is significantly reduced in the presence of even very small amounts of impurities. Therefore, especially pure copper M00 (99.99%), electrolytic copper M0 (99.95%), and M1 (99.9%) are used as conductor materials. Technical copper grades M2 (99.7%), M3 (99.5%), M4 (99.0%).
Depending on the mechanical properties, a distinction is made between hard, cold-worked copper (MT) and soft, annealed copper (MM).
Harmful impurities in copper are bismuth, lead, sulfur and oxygen. The action of bismuth and lead is similar to the action of sulfur in steel; They form low-melting eutectics with copper, located along the grain boundaries, which leads to the destruction of copper when it is processed under pressure in a hot state (the melting point of the eutectic is 270 0C and 326 0C, respectively).
Sulfur and oxygen reduce the ductility of copper due to the formation of brittle chemical compounds Cu2O and Cu2S.
Technically pure copper is rarely used as a structural material, since it has low strength properties and hardness. The main copper-based structural materials are alloys of brass and bronze. To mark copper alloys, use the following letter designation of alloying elements:
- O - tin; C - zinc; X - chromium;
- F - iron; N - nickel; C - lead;
- K - silicon; A - aluminum; F - phosphorus;
- Mts - manganese; Mg – magnesium; B – beryllium.
Brass
Brasses are copper alloys in which the main alloying element is zinc.
Depending on the zinc content, brasses for industrial use are:
- single-phase a - brass containing up to 39% zinc (this is the maximum solubility of zinc in copper);
- two-phase (a+b|)- brass containing up to 46% zinc;
- single-phase b|- brass containing up to 50% zinc.
Single-phase a-brasses are ductile, can be easily processed by cutting and pressure at temperatures below 300 0C and above 700 0C (in the range from 300 0C to 700 0C - the brittle zone). With increasing zinc content, the strength of brass increases. In brasses, the b|-phase is an ordered solid solution based on the electronic connection of CuZn with a bcc lattice; it is brittle and strong. Therefore, the more b|-phase in brass, the stronger and less ductile they are. Brass with a zinc content of up to 42...43% has practical application.
Brasses processed by pressure are marked with the letter L (brass), followed by the letter designations of alloying elements; The numbers following the letters indicate the copper content and the percentage of the corresponding alloying element. The zinc content is determined by the difference from 100%. For example, L62 brass contains 62% Cu and 38% Zn. Casting brass is marked with the letter L, after which the content of zinc and other alloying elements is indicated as a percentage. The amount of copper is determined by the difference from 100%. For example, brass LTs36Mts20S2 contains 36% Zn, 20% Mn, 2% Pb and 42% Cu.
Single-phase a-brasses include L96 (tompak), L80 (semi-tompak), L68, which has the greatest ductility (d = 56%). Two-phase (a+b|) - brasses of grades L59 and L60 have less ductility in the cold state, but greater strength and wear resistance. Single-phase ones have after annealing sв = 250...350 MPa and d = (50...56)%, two-phase ones have sв = 400...450 MPa and d = (35...40%).
To increase the mechanical properties and corrosion resistance of brass, they can be alloyed with tin, aluminum, manganese, silicon, nickel, iron, etc.
The introduction of alloying elements (except nickel) reduces the solubility of zinc in copper and promotes the formation of the b|- phase, therefore such brasses are often two-phase (a+b|). Nickel increases the solubility of zinc in copper, and with sufficient zinc content, brass changes from two-phase to single-phase. Lead facilitates machinability and improves anti-friction properties. Corrosion resistance is increased by Al, Zn, Si, Mn, Ni, Sn.
In marine shipbuilding, tin-bearing “marine” brasses are used, for example, LO70-1 (70% Cu, 1% Sn, 29% Zn). It is used for the manufacture of condenser tubes and parts of heating equipment.
Aluminum brass
used for the manufacture of condenser tubes, tanks, bushings, as well as for the manufacture of corrosion-resistant parts operating in sea water. Brands of brass: LA77-2, LAZ60-1-1, LAN59-3-2 (in electrical machines, in chemical engineering). Solid-drawn round pipes are made from brass LANKMts75-2-2.5-0.5-0.5 for the production of pressure tubes and springs in instruments of a high accuracy class. With the help of hardening and aging, sв reaches 700 MPa.
Manganese brass
in addition to good mechanical and technological properties (processed under pressure in cold and hot conditions), they have high corrosion resistance in sea water, chlorides and superheated steam. Brasses LMts 58-2 and LMtsA 57-3-1 are used mainly for the manufacture of fittings and fasteners.
Silicon brasses
characterized by high strength (sv up to 640 MPa), ductility and toughness up to minus 183 0C. LK80-3 brass is used for the manufacture of fittings and instrument parts in shipbuilding and general mechanical engineering.
Leaded brasses
are perfectly processed by cutting and have high anti-friction properties. Brasses LS60-1, LS59-1 are used for the manufacture of fasteners, gears, bushings.
Nickel brass
has increased mechanical (sv up to 785 MPa) and corrosion properties, processed by pressure in a cold and hot state. Brass LN65-5 is used for the manufacture of manometric and condenser tubes, various types of rolled products.
Foundry brasses
contain the same elements as pressure treated brass; What distinguishes foundries from the latter are, as a rule, greater alloying with zinc and other metals. As a result, they have good casting characteristics.
Answers to crossword of the day No. 21137 from Odnoklassniki
21136 21138
Horizontally: - An alloy called yellow copper - "Colleague" of the magazine "Funny Pictures" - One of the largest cities in Japan - Heat-resistant alloy - Place of torture, prison torture - Legalized freedom - Crowned city of Kazakhstan - Hooligan, friend of Syroezhkin and Electronics - Hut of the Yakuts , covered with birch bark - Bayan with keys instead of buttons - “Mowgli” on the ancient Roman throne - An indispensable characteristic of all skirts - Literary role of Euripides - The connecting part of the skin - The reason for raising the outpost “to the gun!” — Mendeleev identified it as 82nd in a row — River cargo ship — Average between jam and jam — Living on interest from capital
Vertical: - Northern neighbor of Fiji - Appropriation, conquest - German physicist Max .. - Japanese "armored poems" - Countryman of an Ecuadorian, Brazilian and Chilean - Double-action steam engine - Japanese city on which an atomic bomb was dropped - Long gunfire - Master , foreman, concrete worker, mason - Wooden fence, wattle fence - Mexican Panama - Son of the last king of Babylonia - Roman emperor with a “musical” name - One of the soloists of the group “Na-Na” - Long fastening rod - Descendant of Russians and Eskimos
TOMPAK - An alloy of copper and zinc, which has a golden color and is used for the manufacture of cheap jewelry, household items, etc.
NICHROME - An alloy of nickel, iron and chromium that cannot be oxidized at both normal and high temperatures.
DRY - A place of torture, prison torture.
RIGHT cf. 1. A set of norms and rules established and protected by state authorities that regulate the relations of people in society. // A set of laws and regulations of the state related to a certain situation. side of the social structure, life and activities of society. // A set of international agreements, treaties regulating relations between states according to some. questions. // Scientific discipline that studies legal legislation. 2. Freedom, the opportunity to do, implement something, provided by the laws of the state. // Power, powers granted to someone, something. // Advantage, privilege, benefit granted to someone, something. // Permission, permission granted to someone, something. // Official permission, permission to perform something. duties, to do something. positions, rank. 3. Customs existing in some place. environment, in smb. society. 4. The ability to do something, act in some way. way. 5. Reason, reason, reason for something. actions.
ACCORDION - A type of accordion with a piano-type keyboard for the right hand.
LENGTH - 1. The length of a line, plane, body, etc. in the direction in which its two extreme points are farthest from each other. // Extent, distance between the ends of something. 2. Duration, duration (about time).
TRAGICIST - 1. The stage role of an actor playing tragic roles. 2. outdated Author of tragedies.
TRAGIC - 1. One who is distinguished by a tragic attitude.
DERMA - The connective part of the skin in vertebrates and humans, located under the outer layer - the epidermis.
ANXIETY - 1. Strong mental agitation, restlessness, confusion (usually in anticipation of danger, something unknown). 2. Signal of impending danger.
LEAD - 1. A heavy, soft, fusible metal of a bluish-grayish color. 2. transfer Same as: bullet.
BARKA - A non-self-propelled wooden river vessel with steep sides, used until the middle of the 20th century. for transportation of goods.
JAM Wed. A sweet, thick mass of pureed fruits or berries boiled with sugar or molasses.
RANTIER - uncl. One who lives on unearned income received in the form of interest or dividends.
TAKING Wed. The process of action by value. verb: take (1-7,9,10).
PERUAN - see Peruvians.
COMPOUND - 1. A steam engine with two cylinders in which the steam is worked in series. 2. Compound mass.
CANONADA - Frequent and heavy firing from many artillery pieces.
BUILDER - 1. Specialist in the field of construction. 2. The one who builds something is engaged in the construction of something. 3. transfer One who creates something.
TOWN - up-down 1. Action according to value. verb: to fence. 2. Fence, hedge, fence. // Partition, barrier on the river for fishing.
SOMBRERO avg. several Spanish or Latin American wide-brimmed hat.
NAGEL - A large wooden or metal nail used to fasten parts of wooden structures.
CREOLE - 1. Descendant of the Spanish and Portuguese conquerors in Latin America. 2. Descendant from Russian marriages with Indians, Eskimos and Aleuts in the Aleutian Islands and Alaska in the 18th-19th centuries.
Application area of copper alloys
Copper has low resistivity. This property has provided copper with widespread use in the electrical industry. Conductors, wires, and cables are made from copper. Copper is used in the manufacture of printed circuit boards for various electronic devices. Copper wires are used in electric motors and transformers.
Copper has high thermal conductivity. This ensures its use in the manufacture of cooling and heating radiators, air conditioners, and coolers.
The strength and corrosion resistance of copper served as the basis for the manufacture of pipes from it, which have a wide range of applications: in plumbing, gas and heating systems, in refrigeration equipment, and in air conditioning.
In construction, copper is used in the manufacture of roofs and facade parts of buildings.
The bactericidal properties of copper make it possible to use it in medical institutions as a disinfectant material: in the manufacture of interior parts that people touch the most - door handles, railings, handrails, bed sides, etc.
Copper alloys have no less scope of application.
Bronze (by grade) is used in the production of machine parts: steam and water fittings, critical components, bearings, bushings. Tin wrought bronzes are used to produce meshes used in the pulp and paper industry.
Brass (by grade) is used in the production of machine parts in the field of heating engineering and chemical equipment. Various coils and bellows are made from them. In the automotive industry, brass is used for the manufacture of condenser pipes, pipes, and hardware. In shipbuilding and aircraft manufacturing, brass is also used for the manufacture of parts, condenser pipes, and hardware. Parts of watch mechanisms and printing matrices are made from brass.
Cupronickel MNZhMts is used for the production of condenser tubes for sea vessels operating in the most difficult conditions. Cupronickel MH19 is used for the manufacture of medical instruments, coins, jewelry, and cutlery.
Copper
Soft, ductile metal of pinkish-golden color. Its beauty has attracted people since ancient times, so the first products made from copper were jewelry.
In the presence of oxygen, copper ingots and copper products acquire a reddish-yellow hue due to the formation of a film of oxides. In a humid environment in the presence of carbon dioxide, copper turns greenish.
Copper has high thermal and electrical conductivity, which ensures its use in electrical engineering. Does not change properties over a wide range of temperatures from very low to very high. Not magnetic.
In nature, deposits of copper ore are more often found on the surface than other metals. This allows open-pit mining. Large copper nuggets with high copper purity and copper veins are found. In addition, copper is obtained from the following compounds:
- copper pyrite,
- chalcocite,
- bornite,
- covellin,
- cuprite,
- azurite,
- malachite.
Sources of copper for recycling
Saving resources is an important environmental and technological task. Copper is too valuable an element to be thrown away easily. Therefore, when disposing of household devices and appliances (TVs, refrigerators, computer equipment), you need to cut off all copper-containing elements and take them to recycling collection points. Factories must organize a centralized collection of decommissioned power cables and transformers, electric motors, and other copper-containing parts and devices. There is a certain copper content in spoiled fluorescent lamps, which should also be taken into account when recycling.
Copper and copper alloys, mastered by mankind at the very dawn of civilization, remain popular materials in the technological era, the basis of which is iron. Modern industrial production cannot be imagined without the use of non-ferrous metals. In the future, the need for copper and its alloys will only grow, so it is very important to treat these materials sparingly and use them rationally.
Rate this article:
Rating: 0/5 — 0 votes