What is
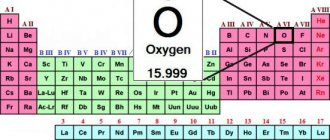
Chromium is a metal, element of the periodic table No. 24.
International designation and formula – Chromium, Cr.
The silvery-bluish shiny substance is one of the hardest (5.5 Mohs) and refractory metals, but is brittle. Refers to ferrous metals.
Its structure and properties are similar to iron, manganese, titanium, and nickel. These elements are combined into one family.
A unique feature of the element is the bright rainbow color of the compounds: blue, violet, green to emerald, yellow, orange, purple. They determined the name, thanks to them it is easy to distinguish chrome from other metals.
In ancient Greek χρῶμα (chroma) - paint, color.
History of the discovery of Chrome Chromium
Discovery of the Chromium element in the Middle Urals, in the Berezovsky gold deposit. It was first mentioned in the work of M.V. Lomonosov “The First Foundations of Metallurgy” (1763) as red lead ore, PbCrO4. The modern name is crocoite. In 1797, the French chemist L.N. Vauquelin isolated a new refractory metal from it (most likely, Vauquelin obtained chromium carbide). He calcined the green oxide Cr2O3 with coal and isolated a refractory metal (with an admixture of carbides). Vauquelin obtained the oxide Cr2O3 by decomposing “Siberian red lead” - the crocoite mineral PbCrO4.
The modern method of obtaining pure chromium (since 1894) differs from the Vauquelin method only in the type of reducing agent. The process of electrolytic coating of iron with chromium was developed in the 20s of the twentieth century.
Physico-chemical characteristics
The physical and chemical properties of chromium are typical of metals:
- Chemically inactive. Under normal conditions it does not interact with water or alkali solutions. The reaction starts at +600°C.
- Oxygen creates a protective oxide film on its surface.
- In compounds it exhibits three degrees: +2, +3, +6. The most stable are trivalent.
The use of chromium is hampered by disadvantages:
- Obvious deterioration of characteristics due to impurities in the composition.
- The need for additional processing of superhard metal to obtain plasticity.
However, they are compensated by the advantages of the metal: refractoriness, hardness (the fifth among metals), and resistance to corrosion.
| Properties of the atom | |
| Name, symbol, number | Chrome / Chromium (Cr), 24 |
| Atomic mass (molar mass) | 51.9961(6) a. e.m. (g/mol) |
| Electronic configuration | [Ar] 3d5 4s1 |
| Atomic radius | 130 pm |
| Chemical properties | |
| Covalent radius | 118 pm |
| Ion radius | (+6e)52 (+3e)63 pm |
| Electronegativity | 1.66 (Pauling scale) |
| Electrode potential | −0,74 |
| Oxidation states | 6, 3, 2, 0 |
| Ionization energy (first electron) | 652.4 (6.76) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 7.19 g/cm³ |
| Melting temperature | 2130 K (1856.9 °C) |
| Boiling temperature | 2945 K (2671.9 °C) |
| Ud. heat of fusion | 21 kJ/mol |
| Ud. heat of vaporization | 342 kJ/mol |
| Molar heat capacity | 23.3 J/(K mol) |
| Molar volume | 7.23 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | cubic body-centered |
| Lattice parameters | 2.885 Å |
| Debye temperature | 460 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 93.9 W/(mK) |
| CAS number | 7440-47-3 |
Being in nature
Two dozen chromium minerals have been identified in nature. The main ones are chromite and crocoite.
Igneous rocks contain different concentrations of the element (g/t):
- ultrabasic – 2000;
- basalts, other basic ones – 200.
Each ton of the earth's crust contains on average 83 g of chromium.
One class of industrial interest is chrome spinels.
The metal contains precious stones - chrome tourmaline, uvarovite (chrome garnet), and others.
Environmental pollution with heavy metals
Elements of the biosphere that are polluted by heavy metals are soil and water. Most often, the culprits for this are metallurgical enterprises that process light and heavy non-ferrous metals. The list of polluting agents also includes waste incineration plants, automobile exhausts, boiler houses, chemical production companies, printing companies, and even power plants.
The most common toxins are: lead (automotive production), mercury (example of distribution: thermometers broken in everyday life and fluorescent lighting fixtures), cadmium (formed as a result of burning garbage). In addition, most factories in production use one or another element that can be characterized as heavy. The metal group, the list of which was given above, most often enters water bodies in the form of waste and then reaches humans along the trophic chain.
In addition to man-made factors of pollution of nature with heavy metals, there are also natural ones - these are volcanic eruptions, in the lava of which an increased content of cadmium was found.
Receiving technology
The traditional raw material for chromium production is chrome spinels.
The main methods of obtaining metal are ore enrichment by electrolysis or reduction.
To increase the purity of the final product, the raw materials are fused in an electric furnace with soda, adding oxygen.
The production of metallic chromium of almost absolute purity is carried out by the electrolysis of concentrated chromium solutions or by the reduction of chromium oxide with aluminum in vacuum furnaces (at 1500°C).
Precautionary measures
Chromium metal and Cr(III) compounds are generally not considered a health hazard, but substances containing Cr(VI) can be toxic if ingested or inhaled. Most of these substances are irritating to the eyes, skin and mucous membranes. With chronic exposure, chromium(VI) compounds can cause eye damage if not treated properly. In addition, it is a recognized carcinogen. The lethal dose of this chemical element is about half a teaspoon. According to the recommendations of the World Health Organization, the maximum permissible concentration of Cr (VI) in drinking water is 0.05 mg per liter.
Because chromium compounds are used in dyes and to tan leather, they are often found in soil and groundwater from abandoned industrial sites requiring environmental cleanup and remediation. Primer containing Cr(VI) is still widely used in the aerospace and automotive industries.
Where is it used?
The metal is used in two ways: as a ligature for other metals and as a coating.
Metallurgy
An industry that takes up three quarters of metal volumes. Steel is alloyed with chromium to improve its quality.
Receive the product:
- stainless;
- wear-resistant;
- heat resistant.
Such advantages of steels led to their use as a material for artillery barrels, submarine hulls, safes, metal-cutting, medical, and chemical instruments. The engines of spaceships and the filling of plasmatrons are made from them.
Even a small amount of chromium in the composition significantly improves the mechanical properties of the material.
The most famous chromium-containing alloys are with nickel (nichrome) and iron (fechral). These are precision materials with increased electrical resistance. Used to work at extreme temperatures.
Other industries
Products made from metal and its alloys are produced for different market segments:
- Bricks are the body of metallurgical furnaces.
- Heating elements (nickel alloy).
- Surgical instruments (alloy with nickel, molybdenum, cobalt).
- Chromium compounds are useful in the production of matches, shoes, clothing (the famous shiny-durable chrome leather), dyeing textiles, and processing furniture wood.
- Green chrome paint is applied to the ceramic before glazing and firing.
Paints made from ground chrome ores were also used by icon painters of Ancient Rus'.
- Trivalent metal oxide is the starting material for growing synthetic rubies for lasers.
- The green fireworks lights are thanks to chrome.
Chrome is purchased by pharmaceutical giants, manufacturers of dietary supplements, and weight loss drugs.
Decor
Chrome plating on a watch case or car parts is not only a status marker. This treatment protects against wear, corrosion, and mechanical damage.
The thickness of the metal coating depends on the purpose of the product: from 2 microns (decorative assortment) to 0.1 mm (parts of bikes, bicycles, cars).
The process of coating with chromium is called chrome plating. It is technologically simple and inexpensive.
Potassium dichromate
K2Cr2O7 is a powerful oxidizing agent and is preferred as a means for cleaning laboratory glassware from organic matter. For this purpose, its saturated solution in concentrated sulfuric acid is used. Sometimes, however, it is replaced by sodium bichromate, based on the latter's higher solubility. In addition, it can regulate the oxidation process of organic compounds, converting primary alcohol into aldehyde and then into carbon dioxide.
Potassium dichromate can cause chrome dermatitis. Chromium is likely to cause sensitization leading to the development of dermatitis, especially of the hands and forearms, which is chronic and difficult to cure. Like other Cr(VI) compounds, potassium bichromate is carcinogenic. It must be handled with gloves and appropriate protective equipment.
Meaning for humans
Chromium is present in the human body initially.
Health
He is a participant in a number of biological processes:
- Lipid, carbon metabolism.
- Removing “bad” cholesterol
- Blood sugar balance.
- Strengthening bone tissue.
- Activation of insulin action.
- Ability to replace iodine.
- Stimulation of tissue regeneration.
A sufficient level of chromium in the body is critical for people with excess weight, diabetes, diseases of the thyroid gland, heart, and blood vessels.
Nutrition
Products from all main groups are rich in chromium:
- Meat – chicken, beef (and liver);
- Fish – mackerel, tuna, herring.
- Cereals – semolina, pearl barley.
- Vegetables – tomatoes, radishes, green onions.
Cheeses, legumes, corn oil, fruits, coarse bread, and brewer's yeast are saturated with metal.
Dosage
Daily requirement for chromium (mcg):
- Children – 12-34 (depending on age).
- Women – 55-68.
- Men – 59-79.
During pregnancy in women, an active lifestyle, and physical activity in men, the need doubles.
General information:
| 100 | General information | |
| 101 | Name | Chromium |
| 102 | Former name | |
| 103 | Latin name | Chromium |
| 104 | English name | Chromium |
| 105 | Symbol | Cr |
| 106 | Atomic number (number in table) | 24 |
| 107 | Type | Metal |
| 108 | Group | Amphoteric, transitional, ferrous metal |
| 109 | Open | Louis-Nicolas Vauquelin, France , 1797 |
| 110 | Opening year | 1797 |
| 111 | Appearance, etc. | Hard bluish-white metal |
| 112 | Origin | Natural material |
| 113 | Modifications | |
| 114 | Allotropic modifications | |
| 115 | Temperature and other conditions for the transition of allotropic modifications into each other | |
| 116 | Bose-Einstein condensate | 52Cr |
| 117 | 2D materials | |
| 118 | Content in the atmosphere and air (by mass) | 0 % |
| 119 | Content in the earth's crust (by mass) | 0,014 % |
| 120 | Content in seas and oceans (by mass) | 6,0·10-8 % |
| 121 | Content in the Universe and space (by mass) | 0,0015 % |
| 122 | Abundance in the Sun (by mass) | 0,002 % |
| 123 | Content in meteorites (by mass) | 0,3 % |
| 124 | Content in the human body (by weight) | 3,0·10-6 % |