Humanity has learned to use gaseous substances to support artificial processes and reactions, as a result of which it is possible to obtain other chemical compounds. In addition, various gases are used to obtain certain physical phenomena and properties. Carbon dioxide or CO2 has a large number of qualities that cannot but be used in the chemical industry and everyday life.
What is carbon dioxide
At atmospheric pressure and room temperature, carbon dioxide is in a gaseous state. This is its most common form, in which it participates in the processes of respiration, photosynthesis and metabolism of living organisms.
Carbon dioxide
When cooled to -78 °C, it, bypassing the liquid phase, crystallizes and forms the so-called “dry ice”, which is widely used as a safe refrigerant in the food and chemical industries and in street trade and refrigerated transportation.
Under special conditions—pressure of tens of atmospheres—carbon dioxide transforms into a liquid state of aggregation. This occurs on the seabed, at a depth of over 600 m.
Refrigeration and freezing of food
Along with liquid nitrogen, liquid carbon dioxide is most suitable for direct contact freezing of various types of products. As a contact refrigerant, it is attractive due to its low cost, chemical inertness and thermal stability, does not cause corrosion, is not flammable, and is not dangerous to personnel.
The use of CO2 in contact quick-freezers provides a number of fundamental advantages compared to traditional freezing technologies: freezing time is reduced to 5 - 30 minutes; Enzymatic activity in the frozen product quickly ceases; the structure of tissues and cells of the product is well preserved, since ice crystals are formed of much smaller sizes and almost simultaneously in the cells and in the intercellular space of tissues; with slow freezing, traces of bacterial activity appear in the product, while with shock freezing with carbon dioxide they simply do not have time to develop; weight loss of the product as a result of shrinkage is only 0.3 - 1% versus 3 - 6%; Easily volatile valuable aromatic substances are retained in large quantities.
Compared to freezing with liquid nitrogen, when using carbon dioxide, there is no cracking of the product due to too large a temperature difference between the surface and the core of the product being frozen; During the freezing process, CO2 penetrates into the product and therefore protects it from oxidation and the development of microorganisms during defrosting.
Fruits and vegetables subjected to quick freezing and packaging on site most fully retain their taste and nutritional value, all vitamins and biologically active substances, which makes it possible to widely use them in the production of products for children's and dietary foods.
Carbon dioxide is often used to quickly cool packaged and unpackaged fresh food products to 2 - 6 °C, which improves the natural color of the product due to the slight diffusion of CO2 into the product. In addition, the shelf life of products is significantly increased, since CO2 suppresses the development of both aerobic and anaerobic bacteria and molds.
In the refrigeration industry, CO2 is used as an alternative refrigerant. Carbon dioxide is an effective refrigerant because it has a low critical temperature (+31.1 °C), a relatively high triple point temperature (-56 °C), high triple point pressure (0.5 MPa) and high critical pressure (7. 39 MPa). As a refrigerant, CO2 has the following advantages: very low cost compared to other refrigerants; non-toxic, non-flammable and non-explosive; compatible with all electrical insulating and structural materials; does not destroy the ozone layer; makes a (specific) moderate contribution to the increase in the greenhouse effect compared to modern halogenated refrigerants.
Properties of carbon dioxide
In the 17th century, Jean-Baptiste Van Helmont from Flanders discovered carbon dioxide and determined its formula. A detailed study and description was made a century later by the Scot Joseph Black. He studied the properties of carbon dioxide and conducted a series of experiments in which he proved that it is released during the respiration of animals.
The substance molecule contains one carbon atom and two oxygen atoms. The chemical formula of carbon dioxide is written as CO2
Under normal conditions it has no taste, color or smell. Only by inhaling a large amount of it does a person feel a sour taste. It is produced by carbonic acid, which is formed in small doses when carbon dioxide is dissolved in saliva. This feature is used for making carbonated drinks. Bubbles in champagne, prosecco, beer and lemonade are carbon dioxide formed as a result of natural fermentation processes or added artificially to the drink.
Physical properties of carbon dioxide
Carbon dioxide is denser than air, so in the absence of ventilation it accumulates below. It does not support oxidative processes such as respiration and combustion.
Therefore, carbon dioxide is used in fire extinguishers. This property of carbon dioxide is illustrated using a trick - a burning candle is lowered into an “empty” glass, where it goes out. In reality the glass is filled with CO2.
Main applications of CO 2:
- in mechanical engineering and construction (for welding, etc.);
- for cold landing of machine parts;
- in fine sharpening processes;
- for electric welding, based on the principle of protecting molten metal from the harmful effects of atmospheric air;
- in metallurgy;
- blowing carbon dioxide gas through molds;
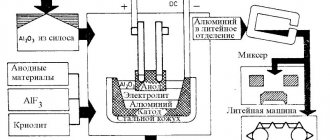
- in the production of aluminum and other easily oxidized metals;
- in agriculture to create artificial rain;
- in ecology it replaces strong mineral acids to neutralize alkaline waste water;
- in the manufacture of fire-fighting means;
- used in carbon dioxide fire extinguishers as a fire extinguishing agent, effectively stopping the combustion process;
- in perfumery in the manufacture of perfumes;
- in the mining industry;
- using the method of flameless rock explosion;
- in the food industry;
- used as a preservative and indicated on the packaging with code E290;
- as a dough leavening agent;
- for the production of carbonated drinks;
Carbon dioxide in nature natural sources
These sources include oxidative processes of varying intensity:
- Respiration of living organisms. From the school course in chemistry and botany, everyone remembers that during photosynthesis, plants absorb carbon dioxide and release oxygen. But not everyone remembers that this happens only during the day, with a sufficient level of lighting. In the dark, plants, on the contrary, absorb oxygen and release carbon dioxide. So trying to improve the air quality in a room by turning it into thickets of ficus and geraniums can play a cruel joke.
- Eruptions and other volcanic activity. CO2 is emitted from the depths of the Earth's mantle along with volcanic gases. In the valleys near the sources of eruptions there is so much gas that, accumulating in the lowlands, it causes suffocation of animals and even people. There are several known cases in Africa when entire villages were suffocated.
- Combustion and rotting of organic matter. Combustion and rotting are the same oxidation reaction, but occurring at different rates. Carbon-rich decaying organic matter from plants and animals, forest fires and smoldering peatlands are all sources of carbon dioxide.
- The largest natural reservoir of CO2 is the waters of the world's oceans, in which it is dissolved.
Carbon dioxide in nature
Over millions of years of evolution of carbon-based life on Earth, many billions of tons of carbon dioxide have accumulated in various sources. Its immediate release into the atmosphere will lead to the death of all life on the planet due to the impossibility of breathing. It’s good that the probability of such a one-time release tends to zero.
Laboratory methods of obtaining
High-quality CO2 is the result of fermentation of alcohol-containing liquids. The gas thus obtained is treated with reagents to form carbon dioxide in the form of water.
In laboratory conditions, a small part of CO2 is extracted. During reactions involving hydrocarbonates and acids. Carbon dioxide is a by-product of reactions in production plants for the extraction of oxygen and nitrogen. CO2 is stored in cylinders at enterprises, as well as in warehouses. The substance is transported in them.
Recommended reading:
- What is the pressure in the carbon dioxide cylinder?
- How much carbon dioxide is used in welding?
Artificial sources of carbon dioxide
Carbon dioxide also enters the atmosphere as a result of human activity. The most active sources in our time are considered to be:
- Industrial emissions occurring during fuel combustion in power plants and technological installations
- Exhaust gases from internal combustion engines of vehicles: cars, trains, airplanes and ships.
- Agricultural waste - rotting manure in large livestock complexes
In addition to direct emissions, there is also an indirect human impact on the CO2 content in the atmosphere. This is massive deforestation in the tropical and subtropical zones, primarily in the Amazon basin.
Artificial source of carbon dioxide
Despite the fact that the Earth's atmosphere contains less than a percent of carbon dioxide, it has an increasing effect on climate and natural phenomena. Carbon dioxide contributes to the so-called greenhouse effect by absorbing the planet's thermal radiation and retaining this heat in the atmosphere. This leads to a gradual but very threatening increase in the average annual temperature of the planet, melting of mountain glaciers and polar ice caps, rising sea levels, flooding of coastal regions and deterioration of the climate in countries far from the sea.
It is significant that against the backdrop of general warming on the planet, there is a significant redistribution of air masses and sea currents, and in some regions the average annual temperature does not increase, but decreases. This gives a trump card to critics of the global warming theory, who accuse its supporters of falsifying facts and manipulating public opinion in favor of certain political centers of influence and financial and economic interests.
Humanity is trying to take control of the carbon dioxide content in the air; the Kyoto and Paris protocols were signed, imposing certain obligations on national economies. In addition, many leading automakers have announced that they will phase out models with internal combustion engines by 2020-25 and switch to hybrids and electric vehicles. However, some of the world's leading economies, such as China and the United States, are in no hurry to fulfill old and take on new obligations, citing a threat to the standard of living in their countries.
Receipt in industry
The production of carbon dioxide in industry is methodologically diverse. It is found in smoke waste released into the atmosphere by thermal power plants and power plants; it is obtained during the fermentation of alcohol and acts as a reaction product with natural carbonates.
The carbon dioxide production industry is broad. Gas can be absorbed in several ways from a single source. In all cases, this is a step-by-step process of removing impurities (to achieve GOST requirements) and achieving the desired consistency and state of aggregation.
Production of carbon dioxide gas
CO2 gas is recovered from industrial (petroleum) fumes by adsorption of monoethanolamine (commercially viable) and potassium carbonate (rare). The principle of collecting carbon particles is the same for both substances. They are sent through a pipeline to the waste and collect carbon dioxide. After collection, carbon dioxide-saturated gases are sent for purification.
In special containers, the reaction occurs at elevated temperatures or low pressure. The process releases pure carbon dioxide and decomposition products (ammonia and others).
Carbon dioxide extraction plant
Schematically the process looks like this:
- The exhaust smoke is mixed with adsorbents (gaseous potassium carbonate or monoethanolamine);
- The gases that have accumulated carbon dioxide enter a special gas holder for purification;
- In a reaction with high temperature or low pressure, carbon dioxide is separated from the adsorbent.
It is not possible to extract much CO2 in the laboratory. But this is possible in a reaction with bicarbonates and acids. Separately, CO2 can be separated in industrial machines to produce oxygen, argon or nitrogen. Carbon dioxide acts as a by-product here. It is stored in special cylinders supplied to the consumer.
Obtaining liquid carbon dioxide
The production of liquid carbon dioxide is associated in stages with its production from gas. From the volatile gaseous state, when treated with hydrogen, a solution of potassium permanganate and coal, liquid dioxide is formed.
Liquefaction occurs due to the low pressure accompanying the reaction. After multi-stage cleaning, liquid carbon dioxide enters the compressor. There it is compressed and fed into 2 adsorbers for drying, which alternately take over the work for recovery. At the same time, the compressed liquid is cleared of odors and transferred to a condenser, and from there for storage.
This liquefaction method is used for alcoholic fermentation gases. It is relevant for propane, butane, etc. It is used in large breweries, and the resulting purified carbon dioxide has high quality indicators.
Production of solid carbon dioxide
Solid dioxide is formed from liquid by treatment at low temperature (-56°). Under industrial conditions, only 20% turns into a solid state, and the rest evaporates.
Dry ice
The procedure for extracting carbon dioxide crystals (dry ice):
- From the fermentation tank, the gas passes into the washing tank;
- In the gas holder after washing it is compressed and liquefied;
- Repeatedly compressed and heated, gaseous carbon is cooled in special refrigerators;
- The liquid is purified with activated carbon;
- It goes into the refrigerator, where it is cooled and further cleaned of impurities;
- The cooled CO2 is sent to the evaporation and press, where the dry ice is assembled.
Carbon dioxide and us: why CO2 is dangerous
Carbon dioxide is one of the metabolic products in the human body. It plays a large role in controlling breathing and blood supply to organs. An increase in CO2 levels in the blood causes blood vessels to dilate, thus being able to transport more oxygen to tissues and organs. Likewise, the respiratory system is forced to become more active if the concentration of carbon dioxide in the body increases. This property is used in ventilators to stimulate the patient’s own respiratory organs to greater activity.
In addition to the benefits mentioned above, excess CO2 concentrations can also cause harm to the body. Increased levels in the inhaled air lead to nausea, headache, suffocation and even loss of consciousness. The body protests against carbon dioxide and sends signals to the person. With a further increase in concentration, oxygen starvation, or hypoxia, develops. Co2 prevents oxygen from joining hemoglobin molecules, which move bound gases through the circulatory system. Oxygen starvation leads to decreased performance, weakened reactions and abilities to analyze the situation and make decisions, apathy and can lead to death.
Common symptoms of carbon dioxide poisoning
Such concentrations of carbon dioxide, unfortunately, are achievable not only in cramped mines, but also in poorly ventilated school classrooms, concert halls, office spaces and vehicles - anywhere where a large number of people accumulate in a confined space without sufficient air exchange with the environment.
Application
According to some estimates, CO2 consumption on the world market exceeds 20 million metric tons per year. Such a high level of consumption is formed under the influence of the requirements of the food industry and oilfield enterprises, carbonation technologies for drinks and other industrial needs, for example, a decrease in the pH value of water treatment plants, problems of metallurgy (including the use of welding gas), etc.
The consumption of carbon dioxide is steadily growing as the scope of its application expands, which covers tasks from industrial purposes to food production - food preservation, in mechanical engineering from welding production and the preparation of protective welding mixtures to cleaning the surfaces of parts with dry ice granules, in agriculture for fertilizing plants, in the gas and oil industry for fire fighting.
Hazard and toxicity class
Carbon dioxide is a non-explosive, non-flammable, non-combustible substance. In gaseous form it is 1.5 times heavier than air, so it accumulates in mines, tunnels, pits, and inside underground equipment.
When the skin comes into contact with carbon dioxide, the affected area may tingle, redness, a feeling of warmth, even frostbite. Slow warming with cold water leads to progression of the disease by an external source of cold.
Hot water can cause burns to the affected area due to partial/complete loss of temperature sensitivity of the dermis. Contact your doctor immediately!
Reservoirs with CO₂ gas can explode when exposed to high temperatures, impacts and sublimation of liquefied acid, or depressurization of the cylinder.
Carbonated drinks
Carbonation of drinks can occur in one of two ways:
- In the production of popular sweet and mineral waters, a mechanical carbonation method is used, which involves saturating a liquid with carbon dioxide. This requires special equipment (siphons, acratophores, saturators) and cylinders with compressed carbon dioxide.
- With the chemical carbonation method, carbon dioxide is produced during the fermentation process. This way you get champagne wine, beer, bread kvass. Carbon dioxide in soda water is obtained as a result of the reaction of soda with acid, accompanied by the rapid release of carbon dioxide.
CO 2 as welding gas
Since 1960, welding of alloy and carbon steels in a carbon dioxide (CO 2 ) environment that meets the requirements of GOST 8050 has become widespread. Recently, the use of welding gas mixtures of argon and helium has become increasingly widespread in the welding technologies of machine-building enterprises, with many of the most popular gas mixtures include a small amount of active gases (CO 2 or O 2) necessary to stabilize the welding arc. However, when welding carbon and low-alloy steels of the main structural classes at Russian enterprises, the main shielding gas continues to be carbon dioxide CO 2, which is explained by the physical properties of this shielding gas and its availability.