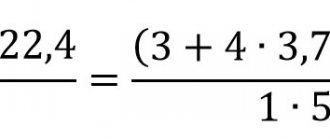Any type of fuel has a set of characteristics that determine its class and quality. One of the main indicators is the specific heat of combustion. This parameter quantitatively expresses the heat released during the combustion of one kilogram of fuel (if we are talking about a liquid or solid product) or 1 cubic meter of fuel (for a gaseous substance).
Why specific heat of combustion is an important characteristic for fuel, what lower heat is and what combustion temperature shows - we’ll talk about this in this article.
Specific heat of combustion of fuel
When completely burned, a certain amount of fuel releases a specific amount of heat. The more heat generated by one kilogram or liter of fuel (in this article we will mainly talk about liquid fuel), the more energy value it has. This means that fuel will be consumed economically.
In physics, the calculation formula is used: Q = q * m, where Q is the amount of heat released in J, q is the specific heat of combustion, expressed in J/m3, m is the mass in kilograms. The higher q, the more energy is obtained during engine operation.
Through complex research processes, the standard specific heat of combustion of most solid, liquid and gaseous fuels has been determined, so q is a tabular value. The specific heat of combustion of the most popular types of liquid mixtures ranges from 43-46 MJ/kg.
The required amount of fuel to operate a 100 W light bulb for a year
(The amount of fuel indicated is below 100% efficiency in converting thermal energy into electrical energy. Since most electric generators and distribution systems achieve efficiency (efficiencies) of about 30% - 35%, the actual amount of fuel used to power a 100 W light bulb will be approximately three times more than the specified amount).
- 166 kg wood
- 117–210 kg of coal
- 73.34 kg kerosene
- 78.8 m³ natural gas (using an average value of 40,000 kJ/m³)
- 58 kg methane
- 0.006 kg uranium
- 17.5 µg antimatter
Heat of combustion lower and higher
Since determining the exact specific heat is a complex process, it is necessary to define the terms used in advance. In our case, we need to separate the low heat of combustion from the higher one.
Higher heat is the amount of heat during complete combustion of fuel, including the precipitation of condensation in the form of water vapor during cooling of substances. The combustion process is accompanied by the release of water due to the content of organic hydrogen in the fuel product; under the influence of high temperature, the water turns into steam. Lower heat does not include condensation of vapors - in this case, condensation is quantified as the latent heat of combustion.
In a research environment, the lower calorific value is taken as 100%, and cooling of the fuel is allowed to the temperature at which steam begins to condense. Everything else is referred to as the latent heat of combustion, which can additionally be over 10%.
It is not considered possible to correctly calculate the lower heat, therefore it is determined by subtracting from the quantitative expression of the higher heat of combustion the numerical expression of the heat obtained from the formation of water vapor of both the fuel itself and the combustion products. The lower heat value is a tabular value and for the main types of fuel is determined by testing.
Since q is defined as a reference value, it becomes easy to compare the appropriateness of using a particular type of fuel in different situations. Thanks to the compiled tables, you can compare the energy efficiency of solid and liquid fuels with their gas equivalent. Thus, one liter of gasoline is comparable in efficiency to 1.3 m3 of gas fuel.
Problem No. 9
The efficiency of the shaft furnace is $60\%$. How much charcoal is needed to heat $10 \space 000 \space kg$ of cast iron from $20 \degree C$ to $1100 \degree C$?
Given: $\eta = 60 \% = 0.6$ $m_h = 10 \space 000 \space kg$ $c_h = 540 \frac{J}{kg \cdot \degree C}$ $t_1 = 20 \degree C$ $ t_2 = 1100 \degree C$ $q_у = 3.4 \cdot 10^7 \frac{J}{kg}$
$m_у — ?$
Show solution and answer
Hide
Solution:
Formula for the efficiency of a shaft furnace: $\eta = \frac{Q _h}{Q_y}$, where $Q_h$ is the amount of heat that must be imparted to the cast iron in order to heat it, $Q_y$ is the amount of heat that will be released during the combustion of wood coal of unknown mass.
Let us express $Q_у$ from here: $Q_у = \frac{Q_ч}{\eta}$.
The amount of heat that will be released during the combustion of charcoal is determined by the formula: $Q_у = q_у m_у$.
Let us express the mass of charcoal from here: $m_у = \frac{Q_у}{m_у}$.
Let us substitute here the found expression for $Q_у$: $m_у = \frac{\frac{Q_ч}{\eta}}{q_у} = \frac{Q_ч}{\eta \cdot q_у}$.
Let us separately calculate the value $Q_h$. The amount of heat required to heat cast iron: $Q_h = = c_h m_h (t_2 - t_1)$.
Let's calculate it: $Q_h = 540 \frac{J}{kg \cdot \degree C} \cdot 10 \space 000 \space kg \cdot (1100 \degree C - 20 \degree C) = 0.54 \cdot 10^7 \ frac{J}{kg} \cdot 1080 \degree C = 583.2 \cdot 10^7 \space J$.
Now we can calculate the required mass of charcoal: $m_у = \frac{583.2 \cdot 10^7 \space J}{0.6 \cdot 3.4 \cdot 10^7 \frac{J}{kg}} = \frac{583.2 \ space kg}{2.04} \approx 286 \space kg$.
Answer : $m_у \approx 286 \space kg$.
Specific heat of combustion of gasoline
The specific heat of combustion of gasoline does not depend on the octane number of the fuel and is determined only by the chemical composition of the product. The more hydrogen compounds it contains, the more moisture and vapor will be formed during combustion and the lower the specific heat will be. This directly reduces the efficiency of the product.
The specific heat of gasoline determined by the research method is 43.5–44.5 MJ/kg. For example, the numerical characteristic for AI-93 gasoline is 43.6 MJ/kg. But for aviation gasoline (B-70 in accordance with GOST) the figure is already 44.1 MJ/kg. This means that B-70 is a more energy efficient fuel.
In practice, it is difficult for a simple car enthusiast to determine the influence of the specific heat of combustion on the operation of a vehicle. However, there are situations in which there is a noticeable decrease in the amount of heat and energy of the fuel. One of them is the presence of mineral compounds and non-combustible residues in the fuel mass. The concentration of the combustible mass decreases, and mineral compounds and ash, which are not subject to combustion, take away part of the released energy.
The presence of a sulfur component in the fuel product also reduces q. During the heating and combustion process, sulfur releases gas, which settles on the internal parts of the working mechanism and enters the human lungs. This leads to the formation of corrosion and premature wear of working elements and pollutes the surrounding air. Therefore, it is very important to choose fuel that is free from most harmful impurities and refuel at proven gas station networks that monitor the reputation of the products they represent.
Brown coal
Brown coal is the youngest solid rock, formed about 50 million years ago from peat or lignite. At its core, it is “immature” coal.
This mineral got its name because of its color - shades vary from brownish-red to black. Brown coal is considered a fuel of low degree of carbonization (metamorphism). It contains 50% carbon, but also a lot of volatile substances, mineral impurities and moisture, so it burns much easier and produces more smoke and a burning smell.
Depending on the humidity, brown coal is divided into grades 1B (humidity more than 40%), 2B (30-40%) and 3B (up to 30%). The yield of volatile substances in brown coals is up to 50%.
With prolonged contact with air, brown coal tends to lose its structure and crack. Among all types of coal, it is considered the lowest quality fuel, since it emits much less heat: the calorific value of combustion is only 4000 - 5500 kcalkg.
Brown coal lies at shallow depths (up to 1 km), so it is much easier and cheaper to mine. However, in Russia it is used much less frequently as a fuel than coal. Due to its low cost, brown coal is still preferred by some small and private boiler houses and thermal power plants.
In Russia, the largest deposits of brown coal are located in the Kansk-Achinsk basin (Krasnoyarsk Territory). In total, the area has reserves of almost 640 billion tons (about 140 billion tons are suitable for open-pit mining).
The only coal deposit in Altai is Soltonskoye, which is rich in brown coal reserves. Its predicted reserves are 250 million tons.
About 2 trillion tons of brown coal are hidden in the Lena coal basin, located on the territory of Yakutia and the Krasnoyarsk Territory. In addition, this type of mineral often occurs together with coal - for example, it is also obtained from the deposits of the Minusinsk and Kuznetsk coal basins.
Specific heat of combustion of kerosene
The chemical structure of kerosene is a straight or branched chain of hydrocarbons; various additives and additives allow this petroleum distillate to be used for the mass supply of vehicles.
In order for the use of kerosene as a fuel to be justified, the selected brand of this combustible mixture must have a maximum specific heat of combustion. In the case of kerosene, the tabular determination of specific heat has an error - due to the variable composition of the fuel, which includes 4 types of hydrocarbons, as a result of which calculations have to be made based on the initial characteristics of the oil used.
It is possible to determine the optimal specific heat of combustion using the minimum combustion temperature of the liquid (+215 degrees) in the calculations. The closer the temperature is to this number, the higher the specific heat capacity of the product, and therefore the higher the specific heat of combustion. Already at +200 degrees, the heat capacity reaches 2900 J/kg*K. Under normal conditions, the specific heat of combustion of kerosene is 43 MJ/kg, with an error of 1000 points in any direction.
The specific heat indicator directly affects the combustion processes of kerosene inside engines. In addition, mechanisms operating on this petroleum product are subject to adiabatic processes due to the direct dependence of the pressure and volume of fuel inside the working chamber. The absence of heat exchange with the external environment leads to maximum energy efficiency of the kerosene used.
Due to the difficulty of determining the exact parameter of the specific heat of combustion of kerosene, to describe the chemical properties of this type of fuel, the specific heat capacity coefficient is preferably used (shows the ratio of the specific heat capacity at a constant volume and pressure level), which also has a constant insignificant error. The physical meaning of accurately calculating these quantities is in the subsequent determination of jet thrust and exhaust speed.
Features of a coal-fired furnace
Such a device has design features that involve the pyrolysis of coal. Charcoal is not a mineral; it has become a product of human activity.
The combustion temperature of coal is 900 degrees, which is accompanied by the release of a sufficient amount of thermal energy. What is the technology to create such an amazing product? The essence lies in a certain processing of wood, due to which a significant change in its structure occurs and excess moisture is released from it. A similar process is carried out in special ovens. The operating principle of such devices is based on the pyrolysis process. A charcoal furnace consists of four basic components:
- combustion chambers;
- reinforced foundation;
- chimney;
- recycling compartment.
Specific heat of combustion of diesel fuel
The higher the specific heat of combustion of a diesel engine, the less volume of liquid is burned during engine operation. Consequently, fuel consumption will be economical. High specific heat is the main criterion for the energy efficiency of diesel fuel.
Thanks to research tests, the tabulated value of the specific heat of combustion of a diesel engine has clear boundaries and amounts to 39.2 – 43.3 MJ/kg. In different countries, the numbers may vary within these two boundaries.
For calculations regarding diesel fuel, only the lower specific heat of combustion is used, which does not include the energy generated during the formation and combustion of hydrogen compounds and the resulting water vapor. The lower specific heat of combustion of diesel fuel is lower than that of alkanes.
The energy efficiency of a diesel engine depends on the degree of viscosity of the liquid. The lower the viscosity, the higher the actual combustion temperature and the higher the lower specific heat of combustion of the fuel.
Chemical stability
Fuel consumption calculator: calculate fuel consumption
When considering the chemical qualities of gasoline, you need to place the main emphasis on how long the composition of hydrocarbons will remain unchanged, since during long-term storage the lighter components disappear and the performance is greatly reduced. In particular, the problem becomes acute if gasoline with a minimum octane number is turned into fuel of a higher grade (AI 95) by adding propane or methane to its composition. Their anti-knock qualities are higher than those of isooctane, but they also dissipate instantly.
According to GOST, the chemical composition of fuel of any brand must remain unchanged for 5 years, subject to storage rules. But in fact, often even newly purchased fuel already has an octane number below the specified one.
Unscrupulous sellers are to blame for this, who add liquefied gas to containers with fuel whose storage time has expired and the contents do not meet the requirements of GOST. Usually, different amounts of gas are added to the same fuel to obtain an octane number of 92 or 95. Confirmation of such tricks is the strong smell of gas at the gas station.
Calorific value of fuel table
Since the specific heat of combustion is a reference value, we present a table with this indicator, determined individually in each case in the laboratory. The table contains information on the main types of fuel used for commercial and industrial purposes.
Table 1
Heat of combustion of fuel
| Name | Specific heat of combustion, MJ/kg |
| Acetylene | 48,3 |
| Hydrogen | 119,83 |
| Propane-butane | 43,8 |
| Isobutane | 45,6 |
| Methane | 50 |
| n-hexane | 45,1 |
| Natural gas | 41…49 |
| Liquefied gas | 45,2 |
| Propane | 46,3 |
| Propylene | 45,8 |
| Ethane | 47,5 |
| AI-72 gasoline | 44,2 |
| AI-93 gasoline | 43,6 |
| Aviation gasoline B-70 | 44,1 |
| Diesel fuel | 43,4 – 43,6 |
| Rocket fuel with kerosene | 9,2 |
| Aviation kerosene | 42,9 |
| Fuel oil | 39 – 41,7 |
| Methanol | 21,1 |
| Butanol-1 | 36,8 |
| Oil | 43,5 – 46 |
| Ethanol | 30,6 |
| Toluene | 40,9 |
#Gasoline#Kerosene#Diesel fuel
Where does the heat come from during the combustion process?
The process of fuel combustion itself is a chemical, oxidative reaction. Most fuels contain large amounts of carbon C, hydrogen H, sulfur S and other substances. During combustion, the C, H, and S atoms combine with O2 oxygen atoms, resulting in molecules CO, CO2, H2O, SO2. In this case, a large amount of thermal energy is released, which people have learned to use for their own purposes.
Rice. 1. Types of fuel: coal, peat, oil, gas.
The main contribution to the heat release is made by carbon C. The second largest contribution to the amount of heat is made by hydrogen H.
Rice. 2. Carbon atoms react with oxygen atoms.
Optimization
An important parameter for optimizing the diesel fuel combustion system in an engine is the proportion of available air participating in this process. The K-factor (the ratio of piston bowl volume to clearance) is an approximate measure of the proportion of air available for combustion. A decrease in engine displacement leads to a decrease in the relative coefficient K and a tendency to deteriorate combustion characteristics. For a given displacement and constant compression ratio, the K-factor can be improved by selecting a longer stroke. The selection of cylinder bore to engine ratio can be influenced by the K factor and a number of other factors such as engine packaging, bores and valves, and so on.
Transportation rules
Transportation of most petroleum products is allowed by all modes of transport: road, rail, air. Special requirements are put forward for containers for petroleum products. They are usually made of aluminum with a protective inner layer or steel. The containers are tightly closed with a lid with a gasket, all conditions are created for complete tightness. The container must be marked with appropriate markings - UN number of the substance, hazard class. Fuel barrels are placed vertically and rigidly fixed. Without obtaining permission from the Ministry of Transport and agreeing on the route, transportation of 1000 liters of gasoline is allowed.
Tanks of road trains must be marked with special markings. The fuel tanker must be equipped with a grounding device. If it is necessary to transport more than 1000 liters of fuel, the driver must have with him:
- route sheet with the indicated place of departure and destination;
- agreement on the transport of dangerous goods;
- permission to transport goods.
The delivery of explosive substances, including hydrocarbon mixtures, can be carried out by trained drivers. They must have a medical certificate. The document confirms the completed stage of medical control. The carrier company must have a permit to transport dangerous goods within the country.
It is important to pay attention to what the operating temperature of the engine should be. Both overheating and decreased performance can significantly damage the system, so it is important to pay attention to this in time and take restoration measures before the breakdown turns into a serious problem, the correction of which will cost a lot of money
Independent heating without mains
Summer diesel fuel: freezing temperature. diesel fuel: characteristics
Its own capacity provides complete control over the fuel supply. You will not be disconnected from the pipeline, you will not be afraid of accidents on the pipeline.
Under any external conditions, the system regularly supplies fuel to the house.
Electricity is also not required - with such a tank you can survive the winter even with a global communications blackout.
Advice:
Choose a container with a reserve - the larger the volume, the less frequently you need to refill it. Keep in mind that the heating season in Russia lasts approximately 7 months (207 days according to SP 131.13330.2012 Construction Climatology).
| House area (m2) | Gas consumption per year (l) | Number of months until gas is completely used up | ||
| Capacity 2.7 m3 (2,295 l) | Capacity 4.85 m3 (4,112 l) | Capacity 9.15 m3 (7,777 l) | ||
| 100 | 3 802 | 7 | 13 | 24.5 |
| 150 | 5 703 | 5 | 8.6 | 16.4 |
| 200 | 7 603 | 3.6 | 6.5 | 12.3 |
| 250 | 9 504 | 3 | 5.2 | 9.8 |
| 300 | 11 405 | 2.4 | 4.3 | 8.2 |
Calculations were carried out using the following indicators:
- a brick house without an attic or basement;
- floor height 3.2 m;
- The efficiency of the gas boiler is 92%.
Calculate yourself
Installation of a 2.5 m3 Real-Invest tank with summer excavation and soil removal (1 machine) costs 198,000 rubles. A model from the same manufacturer for 4.6 m3 with excavation work in summer and soil removal (2 vehicles) - 230,000 rubles. The difference is 32,000 rubles.
The gas tank is filled strictly to 85%. When heating a 100 m2 house, a 2.5 m3 capacity will last for 7 months (2,125 l), and a 4.6 m3 model will last for a year (3,910 l). The difference is 1,785 liters and 5 months of heating.
Refueling and maintenance
Termo Life provides free maintenance of installed gas tanks for 12 months. Then you can extend the service (12,000 rubles per year) or call specialists for a one-time inspection (3,500 rubles).
The optimal mode for refilling the tank is 1-2 times a year. The refill can also be ordered from Termo Life - we supply high-quality “winter” composition regardless of the season.
Temperature
The flame temperature depends on the nature of the combustible substance and the intensity of the oxidizer supply. For example:
- The ignition temperature for most solid materials is 300 °C.
- The flame temperature in a burning cigarette is 250-300 °C.
- Match flame temperature 750-1400 °C; while 300 °C is the ignition temperature of wood, and the combustion temperature of wood is approximately 800–1000 °C.
- The combustion temperature of propane-butane is 800-1970 °C.
- The flame temperature of kerosene is 800 °C, in an environment of pure oxygen – 2000 °C.
- The combustion temperature of gasoline is 1300-1400 °C.
- The flame temperature of alcohol does not exceed 900 °C.
- Magnesium combustion temperature – 2200 °C; a significant part of the radiation is in the UV range.
Highest known combustion temperatures:
- dicyanoacetylene C4N2 5260 K (4990 °C) in oxygen and up to 6000 K (5730 °C) in ozone;
- cyanogen (CN)2 4525 °C in oxygen.
Since water has a very high heat capacity, the absence of hydrogen in the fuel eliminates heat loss due to the formation of water and allows the development of high temperatures.
Ignition temperature of diesel fuel
When considering the ignition temperature of diesel fuel, self-ignition is predominantly indicated. For summer fuel it is 310C, while for winter fuel it is 240C. The reason for this difference lies in the operating and storage conditions. In addition, these indicators strongly depend on pressure, because the combustion process of diesel fuel in the engine occurs precisely at pressure, but without an additional source of spark (in gasoline analogues, spark plugs are used).
One of the important nuances is to take into account the ignition delay, because it is she who has a decisive influence on the central frequency. In addition, the minimal delay can significantly reduce emissions of harmful substances into the atmosphere.