By transferring energy to a body, you can transfer it from a solid state to a liquid state (for example, melt ice), and from a liquid state to a gaseous state (turn water into steam).
If a gas gives up energy, it can turn into a liquid, and a liquid, giving up energy, can turn into a solid.
The transition of a substance from a solid to a liquid state is called melting.
To melt a body, you must first heat it to a certain temperature.
The temperature at which a substance melts is called the melting point of the substance.
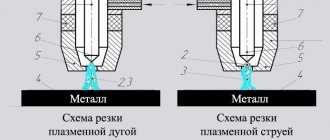
Melting tin in a steel spoon
Some crystalline bodies melt at low temperatures, others at high temperatures. Ice, for example, can be melted by bringing it into the room. A piece of tin or lead - in a steel spoon, heating it on a spirit lamp. Iron is melted in special furnaces where high temperatures are reached.
Table 3 shows the wide range of melting temperatures of various substances.
Table 3. Melting points of some substances (at normal atmospheric pressure)
For example, the melting point of cesium metal is 29 °C, i.e. it can be melted in warm water.
The transition of a substance from a liquid to a solid state is called solidification or crystallization.
For crystallization of a molten body to begin, it must cool to a certain temperature.
The temperature at which a substance hardens (crystallizes) is called the solidification or crystallization temperature.
Experience shows that substances solidify at the same temperature at which they melt. For example, water crystallizes (and ice melts) at 0 ° C, pure iron melts and crystallizes at a temperature of 1539 ° C.
Each metal or alloy has unique properties, including its melting point. In this case, the object passes from one state to another, in a particular case, it becomes liquid from solid. To melt it, you need to apply heat to it and heat it until the desired temperature is reached. At the moment when the desired temperature point of a given alloy is reached, it may still remain in a solid state. As exposure continues, it begins to melt.
Mercury has the lowest melting point - it melts even at -39 °C, tungsten has the highest - 3422 °C. For alloys (steel and others) it is extremely difficult to determine the exact figure. It all depends on the ratio of the components in them. For alloys it is written as a numerical interval.
What is melting point?
Each metal has unique properties, and this list includes its melting point. When melting, the metal changes from one state to another, namely, from solid to liquid. To fuse a metal, you need to bring heat closer to it and heat it to the required temperature - this process is called the melting point. At the moment when the temperature reaches the desired level, it can still remain in a solid state. If you continue the impact, the metal or alloy will begin to melt.
Melting and boiling are not the same thing. The point at which a substance transitions from solid to liquid is often referred to as the melting point of the metal. In the molten state, the molecules do not have a specific arrangement, but attraction holds them close together; in liquid form, the crystalline body leaves volume, but the shape is lost.
During boiling, the volume is lost, the molecules interact very weakly with each other, move chaotically in different directions, and separate from the surface. Boiling point is the process by which the pressure of metal vapor is equal to the pressure of the external environment.
Practical work “Study of the phenomenon of melting and crystallization”
1. Select the substance “Zinc”. Click the "Start" button. The graph shows the process of zinc melting. The boxes on the right display data for a given substance.
Write the data into a table
Initial temperature, °C
Melting point, °C
Melting time, min
Redraw the melting curve for zinc
Click the Reset button to clear all data
2. Choose another substance. Repeat all steps described in step 1
Write the data into a table
Initial temperature, °C
Melting point, °C
Melting time, min
Redraw the melting graph
3. Choose another substance. Repeat all steps described in step 1
Write the data into a table
Initial temperature, °C
Melting point, °C
Melting time, min
Redraw the melting graph
II
.
Study of the “Crystallization” process. Click the "Crystallize" button
1. Select the substance “Zinc”. Click the "Start" button. The graph shows the process of zinc crystallization. The boxes on the right display data for a given substance.
Write the data into a table
Initial temperature, °C
Crystallization temperature, °C
Crystallization time, min
Draw the crystallization graph for zinc
Click the Reset button to clear all data
2. Choose another substance. Repeat all steps described in step 1
Write the data into a table
Initial temperature, °C
Crystallization temperature, °C
Crystallization time, min
Redraw the crystallization graph
3. Choose another substance. Repeat all steps described in step 1
Write the data into a table
Initial temperature, °C
Crystallization temperature, °C
Crystallization time, min
Redraw the crystallization graph
III
.
Write down the conclusion: ______________________________________________________________
IV
. Answer the questions.
To answer the questions, open table No. on page.
Name the most refractory metal. __________________________________________
Name fusible substances? _____________________________________________
Is there a metal that can be melted in your hand? ______________________________
What about in boiling water? __________________________________________________________
Can you melt silver in an aluminum spoon? ___________________________
Is it possible to melt amber in a zinc vessel? _______________________________
Metal stoves are used to heat small rooms. The temperature in the oven reaches 1100 ºС. Is it possible to make this stove out of aluminum? of steel? __________________________________________________________________________
Why do people in the North use alcohol rather than mercury thermometers to measure low temperatures? ______________________________________________
Concept of temperature scale
Some non-metallic objects also have similar properties. The most common is water. A temperature scale was developed regarding the properties of the liquid that occupies a dominant position on Earth. The reference points are the temperature of changes in the aggregative states of water:
- Transformations from liquid to solid and vice versa are taken to be zero degrees.
- Boiling (vapor formation inside a liquid) at normal atmospheric pressure (760 mm Hg) is taken to be 100 ⁰C.
In addition to the Celsius scale, in practice temperature is measured in degrees Fahrenheit and on the absolute Kelvin scale. But when studying the properties of metal objects, other scales are used quite rarely.
Metal melting process
When a part is exposed to heat, a change in the internal structure occurs due to the accumulation of energy by molecules. The speed of their movement increases. At the critical heating point, the destruction of the crystalline structure begins; intermolecular bonds can no longer hold molecules at lattice sites. Instead of oscillatory movements within the unit, chaotic movement occurs, and a melt pool is formed at the heating site. The starting point of melting of a substance in laboratory conditions is determined to hundredths of a degree, and this indicator does not depend on the external pressure on the workpiece. In a vacuum and under pressure, metal workpieces begin to melt at the same temperature, this is explained by the process of accumulation of internal energy necessary for the destruction of intermolecular bonds.
What determines the melting point of a metal?
For different substances, the temperature at which the structure is completely reconstructed to the liquid state is different. If we take into account metals and alloys, then it is worth noting the following points:
- Metals are not often found in their pure form. The temperature directly depends on its composition. As an example, we will indicate tin, to which other substances (for example, silver) can be added. Impurities make the material more or less resistant to heat.
- There are alloys that, due to their chemical composition, can transform into a liquid state at temperatures above one hundred and fifty degrees. There are also alloys that can “hold” when heated to three thousand degrees and above. Taking into account the fact that when the crystal lattice changes, the physical and mechanical qualities change, and operating conditions can be determined by the heating temperature. It is worth noting that the melting point of a metal is an important property of a substance. An example of this is aviation equipment.
Heat treatment, in most cases, almost does not change the resistance to heat. The only sure way to increase resistance to heat is to make changes to the chemical composition; this is why steel is alloyed.
Classification of metals by melting point
In physics, the transition of a solid into a liquid state is characteristic only of substances with a crystalline structure. The melting point of metals is often indicated by a range of values; for alloys, it is difficult to accurately determine heating to the boundary phase state. For pure elements, every degree matters, especially if these are fusible elements,
doesn't matter. The summary table of t indicators is usually divided into 3 groups. In addition to low-melting elements, which heat up to a maximum of +600°C, there are refractory elements that can withstand heating above +1600°C, and medium-melting ones. This group includes alloys that form a melt pool at temperatures from +600 to 1600°C.
Depending on the melting temperature, the melting apparatus is also selected . The higher the indicator, the stronger it should be. You can find out the temperature of the element you need from the table.
Another important quantity is the boiling point. This is the value at which the process of boiling liquids begins; it corresponds to the temperature of saturated steam that forms above the flat surface of the boiling liquid. It is usually almost twice the melting point.
Both values are usually given at normal pressure. directly proportional to each other .
- As the pressure increases , the amount of melting increases.
- As the pressure decreases , the amount of melting decreases.
Different substances have different melting points. Theoretically, metals are divided into:
- Low-melting - temperatures up to 600 degrees Celsius are sufficient to obtain a liquid substance.
- Medium melting - requires a temperature of 600 to 1600 ⁰C.
- Refractory are metals that require temperatures above 1600 ⁰C to melt.
Table of low-melting metals and alloys (up to 600 Co)
Below is a table with the names of low-melting metals and alloys with a melting point of up to 600 Co.
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Tin | Sn | 232 Co | 2600 So |
| Lead | Pb | 327 Co | 1750 So |
| Zinc | Zn | 420 Co | 907 Co |
| Potassium | K | 63.6 Co | 759 Co |
| Sodium | Na | 97.8 Co | 883 Co |
| Mercury | Hg | — 38.9 Co | 356.73 Co |
| Cesium | Cs | 28.4 Co | 667.5 Co |
| Bismuth | Bi | 271.4 Co | 1564 Co |
| Palladium | Pd | 327.5 Co | 1749 Co |
| Polonium | Po | 254 Co | 962 Co |
| Cadmium | Cd | 321.07 Co | 767 Co |
| Rubidium | Rb | 39.3 Co | 688 Co |
| Gallium | Ga | 29.76 So | 2204 Co |
| Indium | In | 156.6 Co | 2072 Co |
| Thallium | Tl | 304 Co | 1473 Co |
| Lithium | Li | 18.05 So | 1342 Co |
Table of medium-melting metals and alloys (from 600С to 1600С)
Below is a table with the names of medium-melting metals and alloys with a melting point from 600 Co to 1600 Co.
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Aluminum | Al | 660 Co | 2519 So |
| Germanium | Ge | 937 Co | 2830 Co |
| Magnesium | Mg | 650 Co | 1100 So |
| Silver | Ag | 960 Co | 2180 Co |
| Gold | Au | 1063 Co | 2660 Co |
| Copper | Cu | 1083 Co | 2580 Co |
| Iron | Fe | 1539 So | 2900 So |
| Silicon | Si | 1415 Co | 2350 Co |
| Nickel | Ni | 1455 Co | 2913 Co |
| Barium | Ba | 727 Co | 1897 Co |
| Beryllium | Be | 1287 Co | 2471 Co |
| Neptunium | Np | 644 Co | 3901.85 Co |
| Protactinium | Pa | 1572 Co | 4027 Co |
| Plutonium | Pu | 640 Co | 3228 Co |
| Actinium | Ac | 1051 Co | 3198 Co |
| Calcium | Ca | 842 Co | 1484 Co |
| Radium | Ra | 700 Co | 1736.85 So |
| Cobalt | Co | 1495 Co | 2927 Co |
| Antimony | Sb | 630.63 Co | 1587 Co |
| Strontium | Sr | 777 Co | 1382 Co |
| Uranus | U | 1135 Co | 4131 Co |
| Manganese | Mn | 1246 Co | 2061 Co |
| Konstantin | 1260 So | ||
| Duralumin | Alloy of aluminum, magnesium, copper and manganese | 650 Co | |
| Invar | Nickel iron alloy | 1425 Co | |
| Brass | Copper and zinc alloy | 1000 Co | |
| Nickel silver | Alloy of copper, zinc and nickel | 1100 So | |
| Nichrome | Alloy of nickel, chromium, silicon, iron, manganese and aluminum | 1400 So | |
| Steel | Iron-carbon alloy | 1300 Co – 1500 Co | |
| Fechral | Alloy of chromium, iron, aluminum, manganese and silicon | 1460 So | |
| Cast iron | Iron-carbon alloy | 1100 Co – 1300 Co | |
Table of refractory metals and alloys (over 1600Co)
Below is a table with the names of refractory metals and alloys with a melting point above 1600 Co.
| Item name | Latin designation | Temperatures | |
| Melting | Boiling | ||
| Tungsten | W | 3420 Co | 5555 Co |
| Titanium | Ti | 1680 So | 3300 So |
| Iridium | Ir | 2447 Co | 4428 Co |
| Osmium | Os | 3054 Co | 5012 Co |
| Platinum | Pt | 1769.3 Co | 3825 Co |
| Rhenium | Re | 3186 Co | 5596 Co |
| Chromium | Cr | 1907 Co | 2671 Co |
| Rhodium | Rh | 1964 Co | 3695 Co |
| Ruthenium | Ru | 2334 Co | 4150 Co |
| Hafnium | Hf | 2233 Co | 4603 Co |
| Tantalum | Ta | 3017 Co | 5458 Co |
| Technetium | Tc | 2157 Co | 4265 Co |
| Thorium | Th | 1750 So | 4788 Co |
| Vanadium | V | 1910 Co | 3407 Co |
| Zirconium | Zr | 1855 Co | 4409 Co |
| Niobium | Nb | 2477 Co | 4744 Co |
| Molybdenum | Mo | 2623 Co | 4639 Co |
| Hafnium carbides | 3890 Co | ||
| Niobium carbides | 3760 Co | ||
| Titanium carbides | 3150 Co | ||
| Zirconium carbides | 3530 Co | ||
What metal can be melted in boiling water?
Boiling and melting points of metals. Melting point of steel
Boiling and melting points of metals
The table shows the melting point of metals tmelt , their boiling point tk at atmospheric pressure, the density of metals ρ at 25°C and thermal conductivity λ at 27°C.
The melting point of metals, as well as their density and thermal conductivity are given in the table for the following metals: actinium Ac, silver Ag, aluminum Al, gold Au, barium Ba, beryllium Be, bismuth Bi, calcium Ca, cadmium Cd, cobalt Co, chromium Cr, cesium Cs, copper Cu, iron Fe, gallium Ga, hafnium Hf, mercury Hg, indium In, iridium Ir, potassium K, lithium Li, magnesium Mg, manganese Mn, molybdenum Mo, sodium Na, niobium Nb, nickel Ni, neptunium Np , osmium Os, protactinium Pa, lead Pb, palladium Pd, polonium Po, platinum Pt, plutonium Pu, radium Ra, rubidium Pb, rhenium Re, rhodium Rh, ruthenium Ru, antimony Sb, tin Sn, strontium Sr, tantalum Ta, technetium Tc, thorium Th, titanium Ti, thallium Tl, uranium U, vanadium V, tungsten W, zinc Zn, zirconium Zr.
According to the table, it can be seen that the melting point of metals varies over a wide range (from -38.83°C for mercury to 3422°C for tungsten). Metals such as lithium (18.05°C), cesium (28.44°C), rubidium (39.3°C) and other alkali metals have a low positive melting point.
The most refractory metals are the following: hafnium, iridium, molybdenum, niobium, osmium, rhenium, ruthenium, tantalum, technetium, tungsten. The melting point of these metals is above 2000°C.
Here are examples of the melting point of metals widely used in industry and everyday life:
- melting point of aluminum 660.32 °C;
- copper melting point 1084.62 °C;
- melting point of lead 327.46 °C;
- melting point of gold 1064.18 °C;
- melting point of tin 231.93 °C;
- the melting point of silver is 961.78 °C;
- The melting point of mercury is -38.83°C.
Rhenium Re has the maximum boiling point of the metals presented in the table - it is 5596°C. Also, metals belonging to the group with a high melting point have high boiling points.
The density of the metals in the table ranges from 0.534 to 22.59 g/cm 3 , that is, the lightest metal is lithium, and the heaviest metal is osmium. It should be noted that osmium has a density greater than that of uranium and even plutonium at room temperature.
The thermal conductivity of metals in the table varies from 6.3 to 427 W/(m deg), thus the worst conductor of heat is a metal such as neptunium, and the best heat-conducting metal is silver.
Melting point of steel
A table of melting temperature values for common grades of steel is presented. Steels for castings, structural, heat-resistant, carbon and other classes of steels are considered.
The melting point of steel ranges from 1350 to 1535°C. The steels in the table are arranged in order of increasing melting point.
What metal can be melted in boiling water?
By transferring energy to a body, you can transfer it from a solid state to a liquid state (for example, melt ice), and from a liquid state to a gaseous state (turn water into steam).
If a gas gives up energy, it can turn into a liquid, and a liquid, giving up energy, can turn into a solid.
The transition of a substance from a solid to a liquid state is called melting.
To melt a body, you must first heat it to a certain temperature.
The temperature at which a substance melts is called the melting point of the substance.
Melting tin in a steel spoon
Some crystalline bodies melt at low temperatures, others at high temperatures. Ice, for example, can be melted by bringing it into the room. A piece of tin or lead - in a steel spoon, heating it on a spirit lamp. Iron is melted in special furnaces where high temperatures are reached.
Table 3 shows the wide range of melting temperatures of various substances.
Table 3. Melting points of some substances (at normal atmospheric pressure)
For example, the melting point of cesium metal is 29 °C, i.e. it can be melted in warm water.
The transition of a substance from a liquid to a solid state is called solidification or crystallization.
For crystallization of a molten body to begin, it must cool to a certain temperature.
The temperature at which a substance hardens (crystallizes) is called the solidification or crystallization temperature.
Experience shows that substances solidify at the same temperature at which they melt. For example, water crystallizes (and ice melts) at 0 ° C, pure iron melts and crystallizes at a temperature of 1539 ° C.
Each metal or alloy has unique properties, including its melting point. In this case, the object passes from one state to another, in a particular case, it becomes liquid from solid. To melt it, you need to apply heat to it and heat it until the desired temperature is reached. At the moment when the desired temperature point of a given alloy is reached, it may still remain in a solid state. As exposure continues, it begins to melt.
Mercury has the lowest melting point - it melts even at -39 °C, tungsten has the highest - 3422 °C. For alloys (steel and others) it is extremely difficult to determine the exact figure. It all depends on the ratio of the components in them. For alloys it is written as a numerical interval.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal applications, resistive heating is used, passing an electric current or induction heating in a high-frequency electromagnetic field . The impact is approximately the same.
At what temperature do metals melt?
Metal elements, whatever they are, melt almost one to one. This process occurs when heated. It can be both external and internal. The first takes place in a furnace, and for the second, resistive heating is used, passing electricity or induction heating. The impact is almost the same. When heated, the amplitude of molecular vibrations increases. Structural lattice defects are formed, which are accompanied by the breaking of interatomic bonds. The process of lattice destruction and the accumulation of such defects means melting.
Melting iron
The melting point of iron is quite high. A technically pure element requires a temperature of +1539 °C. This substance contains an impurity - sulfur, and it can only be extracted in liquid form.
Without impurities, pure material can be obtained by electrolysis of metal salts.
Melting cast iron
Cast iron is the best metal for smelting. The high fluidity rate and low shrinkage rate make it possible to use it more effectively during casting. Next, consider the boiling point of cast iron in degrees Celsius:
- Gray - the temperature can reach 1260 degrees. When pouring into molds, the temperature can rise to 1400.
- White - the temperature reaches 1350 degrees. It is poured into molds at 1450.
The melting rates of a metal such as cast iron are 400 degrees lower compared to steel. This significantly reduces energy costs during processing.
Melting steel
Steel is an alloy of iron mixed with carbon. Its main benefit is strength, since this substance is capable of maintaining its volume and shape for a long time. This is due to the fact that the particles are in an equilibrium position. Thus, the forces of attraction and repulsion between particles are equal.
Steel melts at 1400 °C.
The melting point of stainless steel falls in the middle range between cast iron and steel. Stainless steel is a substance made of alloy steel that has anti-corrosion properties due to the chromium content of 11% or more in its composition.
The melting point of stainless steel ranges from 1,300 to 15,000 °C.
Melting aluminum and copper
The melting point of aluminum is 660 degrees, which means that it can be melted at home.
Pure copper is 1083 degrees, and for copper alloys it ranges from 930 to 1140 degrees.
Melting silver and gold
Silver in its pure form melts at a temperature of 9,620 °C. Moreover, at the melting point of silver, it can be compared with the melting point in degrees of copper alloys.
Gold melts at a temperature of 10,640 °C.
Melting mercury
Mercury has the lowest melting point with a negative value. It is – 38.80 °C.
What metal can be melted in boiling water?
By transferring energy to a body, you can transfer it from a solid state to a liquid state (for example, melt ice), and from a liquid state to a gaseous state (turn water into steam).
If a gas gives up energy, it can turn into a liquid, and a liquid, giving up energy, can turn into a solid.
The transition of a substance from a solid to a liquid state is called melting.
To melt a body, you must first heat it to a certain temperature.
The temperature at which a substance melts is called the melting point of the substance.
Melting tin in a steel spoon
Some crystalline bodies melt at low temperatures, others at high temperatures. Ice, for example, can be melted by bringing it into the room. A piece of tin or lead - in a steel spoon, heating it on a spirit lamp. Iron is melted in special furnaces where high temperatures are reached.
Table 3 shows the wide range of melting temperatures of various substances.
Table 3. Melting points of some substances (at normal atmospheric pressure)
For example, the melting point of cesium metal is 29 °C, i.e. it can be melted in warm water.
The transition of a substance from a liquid to a solid state is called solidification or crystallization.
For crystallization of a molten body to begin, it must cool to a certain temperature.
The temperature at which a substance hardens (crystallizes) is called the solidification or crystallization temperature.
Experience shows that substances solidify at the same temperature at which they melt. For example, water crystallizes (and ice melts) at 0 ° C, pure iron melts and crystallizes at a temperature of 1539 ° C.
Each metal or alloy has unique properties, including its melting point. In this case, the object passes from one state to another, in a particular case, it becomes liquid from solid. To melt it, you need to apply heat to it and heat it until the desired temperature is reached. At the moment when the desired temperature point of a given alloy is reached, it may still remain in a solid state. As exposure continues, it begins to melt.
Mercury has the lowest melting point - it melts even at -39 °C, tungsten has the highest - 3422 °C. For alloys (steel and others) it is extremely difficult to determine the exact figure. It all depends on the ratio of the components in them. For alloys it is written as a numerical interval.
How the process works
Elements, whatever they are: gold, iron, cast iron, steel or any other, melt approximately the same. This occurs due to external or internal heating. External heating is carried out in a thermal furnace. For internal applications, resistive heating is used, passing an electric current or induction heating in a high-frequency electromagnetic field .
The impact is approximately the same. When heating occurs , the amplitude of thermal vibrations of molecules increases. Structural lattice defects appear , accompanied by the rupture of interatomic bonds. The period of lattice destruction and accumulation of defects is called melting.
Depending on the degree at which metals melt, they are divided into:
- low-melting - up to 600 °C: lead, zinc, tin;
- medium-melting - from 600 °C to 1600 °C: gold, copper, aluminum, cast iron, iron and most of all elements and compounds;
- refractory - from 1600 °C: chromium, tungsten, molybdenum, titanium.
Depending on what the maximum degree is, the melting apparatus is selected. It should be stronger the stronger the heating.
The second important value is the boiling degree. This is the parameter at which liquids begin to boil. As a rule, it is twice the melting point. These values are directly proportional to each other and are usually given at normal pressure.
If the pressure increases, the amount of melting also increases. If the pressure decreases, then it decreases.
Characteristics table
Metals and alloys are an indispensable basis for forging , foundry, jewelry and many other areas of production. Whatever the craftsman does ( gold jewelry , cast iron fences, steel knives or copper bracelets) , in order to work correctly, he needs to know the temperatures at which this or that element melts.
To find out this parameter, you need to refer to the table. In the table you can also find the boiling degree.
Among the most commonly used elements in everyday life, the melting point indicators are as follows:
- aluminum - 660 °C;
- copper melting point - 1083 °C;
- melting point of gold - 1063 °C;
- silver - 960 °C;
- tin - 232 °C. Tin is often used for soldering, since the temperature of a working soldering iron is exactly 250–400 degrees;
- lead - 327 °C;
- melting point of iron - 1539 °C;
- the melting point of steel (an alloy of iron and carbon) is from 1300 °C to 1500 °C. It varies depending on the saturation of the steel with components;
- melting point of cast iron (also an alloy of iron and carbon) - from 1100 °C to 1300 °C;
- mercury - -38.9 °C.
As is clear from this part of the table, the most fusible metal is mercury, which at positive temperatures is already in a liquid state.
The boiling point of all these elements is almost twice, and sometimes even higher than the melting point. For example, for gold it is 2660 °C, for aluminum - 2519 °C , for iron - 2900 °C, for copper - 2580 °C, for mercury - 356.73 °C.
For alloys such as steel, cast iron and other metals, the calculation is approximately the same and depends on the ratio of components in the alloy.
The maximum boiling point of metals is rhenium - 5596 ° C. The highest boiling point is for the most refractory materials.
There are tables that also indicate the density of metals . The lightest metal is lithium, the heaviest is osmium. Osmium has a higher density than uranium and plutonium when viewed at room temperature. Light metals include: magnesium, aluminum, titanium. Heavy metals include most common metals: iron, copper, zinc, tin and many others. The last group is very heavy metals, these include: tungsten, gold, lead and others.
Which metal has the highest melting point?
Tungsten is the most refractory metal, 3422 °C (6170 °F).
A hard, refractory, fairly heavy material of light gray color that has a metallic sheen. It is difficult to machine. At room temperature it is quite fragile and breaks. The fragility of the metal is associated with contamination with carbon and oxygen impurities.
Technically, pure metal becomes very ductile at temperatures above 400 °C. Demonstrates chemical inertness and is reluctant to react with other elements. It occurs in nature in the form of such complex minerals as: hübnerite, scheelite, ferberite and wolframite.
Tungsten can be obtained from its ore, through complex chemical processing, as a powder. Using pressing and sintering, it is used to create parts of regular shape and bars.
Tungsten is an extremely resistant element to any temperature influences. For this reason, tungsten could not be softened for more than a hundred years. There was no furnace that could heat up to several thousand degrees Celsius. Scientists have been able to prove that this is the most refractory metal. Although there is an opinion that seaborgium, according to some theoretical data, has greater refractoriness, this is only an assumption, since it is a radioactive element and has a short lifespan.
Brass: varieties and melting point
At what temperature does brass melt? Is it worth melting it at home? How does laser cutting of brass occur? These questions were asked by everyone who was faced with the need to make something from an alloy of copper and zinc. The speed of brass melting and the quality of the future product depend on the correctly selected temperature regime. To avoid damage to the material, read the useful information.
Where is brass used?
A non-ferrous metal such as brass is an alloy of copper and zinc (up to 50%) with possible impurities of a small amount of alloying elements. It has high thermal and electrical conductivity, density in the range of 8300-8800 kg/m3 and strength up to 600 MN/m2. Due to these qualities, as well as its attractive golden yellow color, brass is widely used:
- In art. Figurines and busts of famous figures are often made from this material, as it responds well to high temperatures. In addition, in the search for ideal forms, the finished sculpture can always be melted down.
- In interior and exterior design. Stylish light fixtures, mirror frames, and copper and zinc alloy countertops create a 1970s and mid-century modern feel while serving utilitarian functions. To prevent the alloy from blackening when exposed to air, the products are coated with protective compounds.
- In industry. The alloy of copper and zinc has a low coefficient of friction, so it is often used to cover the rubbing surfaces of bearings and other parts, and to produce mechanisms for land and water transport, fittings, etc.
brass products
- In construction. Bronze and brass are resistant to corrosion, so products made from them can be used in conditions of high humidity. Shut-off and balancing brass fittings are common when installing water pipelines.
Types of brass
Depending on the composition of chemical substances, brasses are divided into:
- Two-component, or simple. Such alloys include mainly copper and zinc, the amount of other elements is insignificant. In turn, among them are:
- Alpha brass, or single-phase. They contain less than 39% zinc, so there is no need to bring the melting point to 905 °C for it to dissolve into copper.
- Beta brass, or two-phase. The second phase of brass occurs when the alloy contains more zinc than can be dissolved. As a rule, b-brasses are not as ductile as a-brasses, but are more durable.
classification of brass by chemical composition
- Multicomponent or special. They consist of copper, zinc and alloying elements such as iron, tin, silicon, aluminum, manganese and lead.
According to the degree and quality of brass processing there are:
- Deformable. For the manufacture of parts, such states of deformable brass as extra hard (with compression >50%), hard (with compression >30%), semi-hard (with compression 10-30%) and soft (annealed alloys) are used. A mixture of copper and zinc is presented in the form of round tubes, wire, tapes, sheets.
- Foundries. Cast brass is a low-melting variety that contains at least 50-80% copper, the rest is zinc and alloying elements. This includes the resulting brass products, as well as fittings.
At what temperature does brass melt?
Without knowledge of at what degrees brass melts and how to melt it, it will be impossible not only to cast parts from an alloy of copper and zinc, but also to laser cut brass. An incorrectly selected temperature for processing will lead to deterioration in the quality of the alloy and unnecessary energy consumption.
The melting point of brass is 880-950 °C. This indicator varies depending on the chemical composition of the alloy. The specific heat of fusion of brass does not coincide with the casting temperature. This is especially noticeable when melting lead brasses, which have reduced fluidity. The difference between their melting and casting temperatures is 145-185 °C.
For example, brass grade LS59-1V melts at a temperature of 900 ° C, but casting can be carried out at 1030-1080 ° C. For grades LS59-1 and LS74-3, these figures are 885-895 °C / 1030-1080 °C and 965 ° C / 1120-1160 °C, respectively, etc. For two-component brasses, the melting and casting temperatures are the same.
For example, for L60 it is 885-895 °C, L80 -965-1000 ° C, L96 - 1055-1070 °C.
The specific heat capacity of brass is 380 J/(kg °C). In other words, to heat 380 kg to a temperature of 1 °C, you need to spend 1 J of energy.
processing modes for simple and lead brasses
Please note: the more lead and bismuth in brass, the more difficult it will be to melt. Brass, which contains a large amount of zinc, melts most quickly. Alloys where the amount of this element reaches 32.5% can be processed without heating, using broaching or rolling.
Why is brass melting necessary?
As a rule, brass is melted before it is used to make fittings, condenser pipes, separators, worm screws, bushings, and other parts intended for use at high temperatures (up to 300 degrees Celsius).
Brass is melted to cast railings, cornices, door handles, decorative panels, frames for mirrors and paintings. Kitchen utensils can also be cast from this alloy: teapots, samovars, trays, bread bins, decorative dishes for hanging on the wall.
A mixture of copper and zinc is also useful for making souvenirs and jewelry.
Knowing how to melt brass, you can do this at home. In everyday life, bolsters, backplates, furniture and window fittings, etc. are cast from molten brass.
Melt brass at home
Equipment for melting brass at home is an induction furnace made of refractory materials, a crucible made of graphite or fireclay bricks, a foundry ladle, steel tongs and a volumetric spoon.
Before melting the metal, the crucible must be heated for 20-30 minutes at a temperature of at least 95 °C.
A spoon is needed to remove slag, tongs are needed to remove the crucible from the furnace, and a ladle is needed to support the crucible when pouring metal.
melting brass at home
To ensure safety, the ground should be covered with an asbestos sheet, and the molten metal should be carried to the molds strictly above the sand box. Special equipment is required. To avoid poisoning by toxic substances, the stove should be placed outdoors or in a well-ventilated area.
When the equipment is ready for use, the material to be melted is crushed and placed in a crucible, which is sent to the furnace. The crucible must remain in the furnace until the metal is completely melted.
You can monitor this process through the window if the oven is factory-made, or by periodically lifting the refractory lid if the oven is homemade.
Liquid brass is poured into a mold where it must cool before final processing.
You can also melt brass at home using a gas torch. To do this, it is placed under a container containing the crushed alloy. By uniformly heating the bottom of the container, you can achieve a liquid state of the metal.
Please note that during melting it is necessary to prevent the appearance of even small bubbles, which can spoil the quality of the future product. Molten metal cannot be stirred, even while removing slag from its surface.
Is it possible to solder brass?
brass soldering
Many beginners, as a rule, are concerned with the questions: can brass be soldered or not and to how many degrees can it be heated. The answer is clear: you can solder brass. It is quite possible to solder brass surfaces, although it will require more skill than when joining with conventional solder.
Solder for brass should consist of copper and silver, combined in a ratio of 1 to 2. Parts placed on an asbestos base are moistened with flux (borax, boric acid, water), sprinkled with crushed solder, then heated with a gas burner.
The temperature should not exceed 700° C to avoid deformation of parts; heating should be done gradually.
The difference between the melting temperatures of solder and brass parts does not exceed 50 °C, so if overheated, there is a risk of getting a large ingot instead of a quality product.
If the work was done efficiently, the seam will have the same color as the brass surface of the part. This is explained by chemical diffusion. The last stage of soldering is removing flux residues.
To do this, hot three percent sulfuric acid is used, which is then washed off the product with water.