Formation of pores in welds and ways to prevent them
Home / Library / Welding quality / Formation of pores in welds and methods for their prevention
Pores observed in welds are associated with the processes of gas evolution in macro- and microvolumes.
When the metal of the weld pool is volumetrically supersaturated with gases, caused by a decrease in solubility due to a decrease in the temperature of the metal, macropores are mainly formed. The growth of gas bubbles in this case occurs mainly as a result of convective diffusion of gas from the surrounding volumes of metal. The rate of bubble growth is determined by the degree of supersaturation of the bath with gases and the rate of desorption of gases into the nucleus.
With local supersaturation of the liquid metal at the crystallization front, the nucleation and development of bubbles is most likely at the stage of stopping the growth of crystals. Bubbles in this case mainly develop due to the diffusion of gas atoms (ions) from adjacent microvolumes of metal. The size of the bubbles is determined mainly by the duration of stops in crystal growth. During the crystallization of the first layers and the duration of stops of 0.1...0.2 s, characteristic of the most commonly used welding modes, the formation of small pores is likely at the fusion line. The role of nitrogen in the formation of large pores in the absence of convective gas mass transfer is small.
Obtaining tight welds when welding with coated electrodes and flux-cored wires can be achieved by reducing the gas content in the weld pool below the solubility limit in the solid metal at the melting temperature. In this case, the formation of gas bubbles at the moment of crystallization does not occur. This method of ensuring tight seams is implemented in electrodes with a basic type of coating.
When the electrode coating of the main type is moistened, the hydrogen content in the weld pool increases above its solubility limit in solid iron at the melting temperature and falls into the most dangerous concentration zone of the solubility jump (12... 27 cm3/100 g) from the point of view of pore formation. At such hydrogen concentrations, the process of formation and removal of gas bubbles from the weld pool proceeds sluggishly, which leads to the formation of pores.
The pores found in welds when welding with long arc electrodes coated with carbonate-fluorite are caused by the release of nitrogen. Poor wetting of electrode metal droplets and the bath with slag from electrodes of this type creates conditions for direct contact of the metal with the gas phase and increased nitrogen absorption.
The gas that causes porosity in welds when welding with rutile and ore coated electrodes is mainly hydrogen. The release of carbon monoxide and nitrogen plays a minor role.
Obtaining dense seams when welding with these electrodes is achieved by creating favorable conditions for increased absorption of hydrogen at the droplet stage and intensive growth and rapid removal of formed gas bubbles from the weld pool until it crystallizes. This situation is realized when the hydrogen content in the weld pool is ensured, significantly exceeding the limit of its solubility in liquid iron at the melting temperature, i.e. much more than 27 cm3/100 g.
The introduction of materials containing crystallization moisture into rutile and ore-acid coatings promotes intense absorption of hydrogen by drops of the electrode metal and the high-temperature region of the weld pool, which subsequently creates favorable conditions for the nucleation, growth and removal of gas bubbles until the crystallization of the weld pool.
An increase in current strength when welding with electrodes with rutile and ore-acid coatings increases the likelihood of pores forming in the weld metal, which is caused by overheating of the second half of the electrode, a decrease in the moisture content in the overheated coating and the hydrogen content in the weld metal made by the overheated part of the electrode to a dangerous concentration level (12... 27 cm3/100 g).
When significant amounts of aluminum, titanium, and silicon are introduced into the coatings of rutile and ore acid electrodes, the probability of pore formation increases, due to an increase in the concentration of silicon in the metal of the weld pool.
Being a surface-active element, silicon inhibits the desorption of hydrogen, degassing of the bath is sluggish, and pores form in the metal. Sulfur and other surfactants can have a similar effect.
Deoxidation of coatings of rutile or ore acid electrodes with silicon, titanium, aluminum, carbon, a high content of these elements in the base metal, an increase in the calcination temperature, a decrease in the oxidation potential of the coating, etc. lead to a decrease in the rate of gas evolution and the formation of porosity.
Suppressing the silicon reduction process by increasing the basicity of the slag, introducing carbonates into the coating and oxidizing silicon with water vapor helps to increase the rate of hydrogen evolution. The proposed method for intensifying hydrogen evolution was used to create industrial grades of rutile-carbonate electrodes of the ANO series.
Less protection of the metal from air when welding with open arc flux-cored wires leads to greater (compared to electrodes) absorption of nitrogen by the metal, therefore the release of nitrogen from the bath has a significant, and in some cases decisive, effect on porosity. In wires of the carbonate-fluorite type, preventing the release of nitrogen in the form of a gas phase is achieved by alloying the metal with titanium and aluminum. Nitrogen absorption can be effectively reduced by covering the welding zone with carbon dioxide, argon-based gas mixtures, or using double-layer wire.
| ← Formation of hot cracks during welding | Methods for assessing the weldability of metals and their alloys → |
Share link:
Welding errors and defects that a novice welder needs to know about
Root weld defects - lack of penetration or insufficient penetration of metal along the root of the weld. Most often, this problem occurs as a result of low current for welding or a large diameter of the selected electrode. It is necessary to increase the welding current, but do not overdo it, since you can burn through the root weld.
Fusion defects are a familiar situation to many novice welders, when a seemingly normally welded workpiece falls apart at the seams. This problem occurs due to non-fusion of the base metal with the deposited metal. It's all about the wrong selection of electrodes; they are of insufficient diameter and cannot cope with heating the cold metal. It is necessary to select electrodes of a larger diameter or consider the possibility of heating the metal.
Weld Edge Defects – Another common welding problem is weld edge defects. Melted and uneven, they make the weld joint unsightly. As a rule, the problem lies in excessive welding current parameters, but not always. Often, defects in the seam edges are formed due to the arc being too long, and also when the electrode does not move correctly along the joint.
Types of defects in welded joints, depending on their shape
Existing weld defects can be divided into two types based on their shape. These are plane defects and spatial defects. Planar defects include hot and cold cracks and lack of weld penetration.
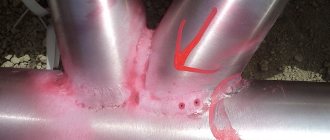
Spatial include various slag inclusions, pores, gas bubbles and all types of incorrectly executed welds (undercuts, burns, incorrect configuration, displacement, etc.).
Planar weld defects pose the greatest danger to the joint as a whole. And the existing types of cracks, depending on the temperature at which they appear, can be divided into several more types, which are mentioned above in the text.
Causes of defects
There are two types of factors affecting the quality of welding work:
- Objective - related to the properties of the materials being welded, the behavior of metals under conditions dictated by the technological process. It is not without reason that one of the important characteristics of any alloy is weldability. Sometimes it becomes necessary to weld materials with poor weldability. Such tasks are sometimes posed in small-scale or single-piece production. Even with full compliance with the requirements of the technological process, a certain percentage of defects may remain, which has to be officially considered acceptable.
- Subjective - depending on the performers. Moreover, the performers should include not only the workers performing welding, but also the technologists who are responsible for the correctness of the technological process parameters, the correct choice of equipment and welding modes.
The main subjective reasons for the occurrence of welding seam defects are:
- errors in preparing welded surfaces;
- use of a tool different from that specified by the technologist;
- welding tool malfunction;
- little work experience and low qualifications of the welder;
- deviation from the required welding conditions.
Poor preparation of metals when welding with argon
A large proportion of errors in argon arc welding also occur due to poor preparation of the metal before welding. For example, if, when welding with argon, oxides are not first removed from the surface of aluminum, then the weld will turn out dirty and with a large number of deep pores.
Therefore, before TIG welding in an argon environment, it is imperative to properly prepare the metals. There should be no dirt or oxide film on the surfaces to be welded.
Control methods
To prevent the occurrence of defects, systematic monitoring must be carried out at all stages of production: before, during the welding process, and after completion.
- Before welding, the preparation of the joining surfaces and their geometry are checked.
- During the process, compliance with all parameters of the technological process, including welding modes, is carefully monitored.
- After welding, the finished product is inspected.
The main methods for identifying weld defects:
- Visual inspection and geometry check. It is suggested that a magnifying glass be used to detect small surface cracks and pores. The metal area is cleaned with sandpaper and etched with a solution of nitric acid. A matte surface is formed, on which cracks are more noticeable. After inspection, the remaining acid is removed.
- Mechanical properties testing. Together with the product, samples are welded and sent to the laboratory to determine tensile strength, relative elongation, and impact strength.
- Macrostructure control. It is carried out on samples that have undergone grinding and etching.
- Microstructure control. Conducted on samples using a microscope. This research method makes it possible to detect burnout, grain boundary oxides, changes in metal structure, and microcracks.
- Hydraulic and pneumatic tests. Used for monitoring vessels and pipelines.
- X-ray control. X-ray scanning makes it possible to identify pores, lack of penetration, cracks, and slag inclusions.
- Ultrasonic testing. Produced using an ultrasonic flaw detector. High-frequency vibrations penetrate the metal and are reflected from cracks, pores and other defects.
- Monitoring for the presence of intergranular corrosion. Carry out only for products exposed to aggressive environments.
Read also: Instruments for inspection of buildings and structures