- Place in the periodic table
Carbon is the most important chemical element of Mendeleev's periodic table. Without him, as well as without
oxygen and hydrogen, Life itself would be unthinkable. It can be said without exaggeration that the life of all living beings, from amoebas to humans, is built precisely from carbon compounds. Carbon is a biogenic element that forms the basis of life on our planet. Being a structural unit of a huge number of different organic compounds, it participates in the construction of living organisms and in ensuring their vital functions. Even the emergence of Life itself is considered by scientists as a complex process of the evolution of carbon compounds. And what are the chemical and physical properties of this wonderful element, the history of its discovery and modern application in chemistry, read about it further.
History of discovery
In fact, carbon has been known to man since ancient times in the form of its allotropic modifications: diamond and graphite. In addition, carbon in the form of charcoal was actively used in metal smelting. The very name of carbon as a chemical element comes from coal.
But in those distant times, people used carbon in the form of coal, or admired it in the form of diamonds, unconsciously, without understanding what important chemical element was behind all this.
The scientific discovery of carbon occurred in 1791, when the English chemist Tennant first obtained free carbon. To obtain carbon, he passed steam
phosphoranad with calcined chalk. As a result of this chemical reaction, calcium phosphate and pure carbon were formed. However, this experiment was preceded by other quests, for example, the outstanding French chemist Lavoisier experimented with burning diamonds using a large incendiary machine. The precious diamond burned without a trace, after which the scientist came to the conclusion that the diamond was nothing more than crystalline carbon.
It is interesting that in these experiments they tried to burn other precious stones, for example, ruby, together with diamond. But other stones withstood high temperatures, only diamond burned without a residue, which drew attention to its excellent chemical nature.
Structure of carbon atoms
There are 6 electrons in a carbon atom, of which 4 electrons are located in the outer electron layer (see Fig. 32):
The carbon atom lacks 4 electrons before completing its outer electron layer. Therefore, in their compounds with metals and hydrogen, carbon atoms exhibit a negative oxidation state of –4, for example, aluminum carbide.
In compounds with more electronegative elements, carbon atoms exhibit positive oxidation states +4 and +2, for example - carbon dioxide and CO - carbon monoxide.
Structure and physical properties of simple substances
Like oxygen, sulfur and phosphorus, carbon forms several allotropic modifications. The most famous of them are graphite and diamond.
Graphite is a dark gray substance consisting of carbon atoms, which are arranged in layers (Fig. 88). These layers are relatively loosely bonded to each other, so the graphite is soft and can be separated into individual flakes. The ability of graphite to leave a line when rubbed is the basis for its widespread use in the production of pencils.
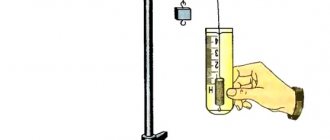
Soot, charcoal, obtained by heating wood without access to air, and coke , obtained from coal, are products with a high carbon content. Charcoal has the ability to absorb (adsorb) vapors, gases and substances from liquid solutions. This is explained by the fact that it has a large number of pores and, therefore, has a large surface area. Place crushed charcoal in a glass with litmus solution (Fig. 89). After some time, the liquid in the glass will become discolored as the charcoal absorbs the litmus.
The sorption properties of charcoal are widely used in gas masks, in the chemical industry, for decolorization and purification of sugar syrup, oil, fats, wines, drinking water, and also in medicine.
Unlike graphite in diamond (Fig. 90), each carbon atom is connected to other atoms by four chemical bonds directed towards the vertices of the tetrahedron. All bonds between carbon atoms are identical, short in length and very strong. Therefore, diamond is the hardest natural substance. Diamond forms transparent crystals that highly refract light. Cut diamonds are called brilliants.
Graphite conducts electricity well, while diamond is an insulator.
Other allotropic modifications of carbon are also known: carbyne, fullerenes, graphene. You will become acquainted with them in the 11th grade chemistry course.
The fact that different allotropic modifications of carbon consist of atoms of the same element can be verified by burning them in oxygen. When burned, they all form the same product - carbon monoxide (IV) and nothing else. In addition, equal masses of graphite, diamond, carbyne and fullerene will give the same amount of carbon dioxide.
At the end of the 18th century. the famous French chemist Lavoisier, together with his colleagues, bought a small diamond and burned it in a huge “incendiary machine” (see figure) using focused solar rays. In this case, only one product was formed - carbon dioxide CO2. Lavoisier obtained the same gas by burning charcoal. These experiments allowed the scientist to conclude that diamond and coal have “the same beginning.”
Under certain conditions, it is possible to transform one modification of carbon into another. Thus, when heated strongly without access to air, the diamond turns black and turns into graphite. Graphite at temperatures above 2000 °C and a pressure of about 100,000 atm turns into diamond. This process is used to produce artificial diamonds that have technical applications.
When writing equations for chemical reactions, various modifications of carbon are designated by the letter C.
Chemical properties of carbon
Carbon reacts with other substances, usually when heated.
The oxidizing properties of carbon manifest themselves when it interacts with metals at high temperatures:
The resulting compounds are called carbides.
Carbon exhibits reducing properties when interacting with oxygen, forming carbon monoxide (II) when there is a lack of oxygen:
or in its excess - carbon monoxide (IV):
The reducing properties of carbon also appear in reactions with complex substances. Thus, by reacting carbon with iron(III) oxide, metallic iron is obtained:
This is one of the very first chemical processes mastered by man.
- Of the allotropic modifications of carbon, the best known are graphite and diamond.
- When interacting with other substances, carbon can exhibit both reducing and oxidizing properties.
Carbon oxides
Among the inorganic compounds of carbon, the most important are its oxygen compounds: oxides, carbonic acid and its salts.
Carbon(II) monoxide
The model of the carbon monoxide molecule is presented in Figure 91. It belongs to non-salt-forming oxides, since it does not interact under normal conditions with either acids or alkalis.
Carbon monoxide (II) CO is formed during incomplete combustion of fuel (wood, peat, coal) and can enter the air. When a person inhales such air, poisoning occurs (fumes), which is why CO is called carbon monoxide. Carbon monoxide is also found in tobacco smoke and car exhaust. Carbon monoxide (II) is a strong poison! When inhaled, it binds to hemoglobin in the blood more strongly than oxygen, thereby blocking the transport of oxygen in the body. Oxygen starvation occurs, accompanied by headache and loss of consciousness. Severe poisoning can result in death. A person affected by carbon monoxide should be taken out into fresh air as quickly as possible and given medical assistance.
Carbon monoxide (II) burns in air with a bluish flame, releasing a large amount of heat, turning into carbon dioxide:
In this reaction, carbon(II) monoxide exhibits reducing properties.
The reducing properties of carbon(II) monoxide are also manifested in its reactions with metal oxides. The products of these reactions are metal and carbon dioxide:
This reaction is used industrially to obtain metals from ores.
Carbon monoxide (II) serves as a starting material for the production of large amounts of organic substances. At the same time, it is one of the most dangerous air pollutants.
Carbon monoxide
You are already familiar with carbon monoxide (IV), or carbon dioxide. The molecular model and graphical formula of this oxide are shown in Figure 92.
The molar mass is approximately 1.5 times the average molar mass of air (29 g/mol), so carbon dioxide is heavier than air. Carbon dioxide does not support breathing, so in its atmosphere animals and humans die from lack of oxygen.
When cooled or under increased pressure, carbon dioxide solidifies, forming a white crystalline substance resembling snow (“dry ice”). In this form, it is widely used as a cooling agent for storing perishable products such as ice cream.
Carbon dioxide can be produced by reacting carbon with oxygen when heated:
Carbon monoxide (IV) is formed by the combustion of various organic substances (methane, alcohol, coal, etc.). The reaction is accompanied by the release of a large amount of heat, so the combustion of these substances is used to produce thermal energy. Carbon dioxide is also formed during the respiration of living organisms and during decay.
In industry, carbon oxide (IV) is produced by burning limestone
In the laboratory it is possible to obtain by the action of acids on carbonates, for example calcium carbonate (Fig. 93):
Carbon dioxide is an acidic oxide; it dissolves slightly in water, forming weak carbonic acid:
This is what causes the sour taste of carbonated and some mineral waters.
With basic oxides and alkalis it forms salts of carbonic acid - carbonates:
When passing calcium hydroxide (limewater) through a solution, the solution becomes cloudy and a precipitate of calcium carbonate precipitates (Fig. 94):
This reaction is qualitative to carbon dioxide.
Carbon(II) monoxide is characterized by reducing properties.
Carbon(IV) monoxide - Carbon dioxide is an acidic oxide. When dissolved in water it forms weak carbonic acid.
Carbon dioxide reacts with basic oxides and bases.
Carbonic acid and its salts
In a carbonic acid , the carbon atom is connected to three oxygen atoms by one double bond and two single C-OH bonds. The molecule model and graphical formula of carbonic acid are presented in Figure 95.
In aqueous solutions, carbonic acid is a very fragile substance. When you try to isolate it from solution, it almost completely decomposes into carbon dioxide and water:
At the same time, the solution in water tastes slightly sour, and when litmus is added to the solution, it turns pink. Therefore, a solution of carbon monoxide (IV) in water can be considered a solution of carbonic acid.
In 2011, researchers from the Technical University of Vienna and the University of Innsbruck (Austria) obtained carbonic acid in the form of a white solid that is stable in air at temperatures below –30 °C.
Carbonic acid is a weak dibasic acid and dissociates stepwise in an aqueous solution. At the first stage of dissociation, a hydrogen ion and a bicarbonate ion are formed
The prefix hydro- in the name of an acidic residue indicates the presence of a hydrogen atom in its composition. Salts containing such an acidic residue belong to the so-called acid salts and are called bicarbonates .
In the second step, the bicarbonate ion dissociates to form a hydrogen ion and a carbonate ion
Salts containing the carbonate ion are medium and are called carbonates .
Chemical properties of carbonic acid salts
Salts of carbonic acid, except for the carbonates of most alkali metals, decompose when heated to release carbon dioxide:
Carbonates and bicarbonates, as salts of a very weak acid, react with all stronger acids to release carbon dioxide. If you drop a solution of hydrochloric acid onto a piece of chalk, which is calcium carbonate, you will see a characteristic effervescence due to the rapid release of carbon dioxide (Fig. 96):
This test can be carried out with both solid carbonates and their solutions. The above reaction is considered as a qualitative reaction for the determination of carbonate ions.
For soluble carbonates, the equation for the qualitative reaction to ions can be written in abbreviated ionic form:
Carbonates can be used to neutralize acids, since when they interact with acids, hydrogen ions bind. For example, ground limestone, consisting mainly of CaCO3, and dolomite flour (CaCO3 MgCO3) are added to soils when they are too acidic. Wood ash plays a similar role due to the potassium carbonate it contains.
Transformations of carbonates and bicarbonates
If you pass carbon dioxide through a solution of calcium hydroxide (see Fig. 94), then clouding of the solution will be observed due to precipitation:
With further passage of carbon dioxide, the calcium carbonate solids will dissolve and the liquid will become clear again. Water-soluble is formed :
When heated, calcium bicarbonate turns into carbonate:
In nature, the occurrence of processes involving carbon dioxide, water and limestone, chalk, marble (all these substances are CaCO3 in chemical composition) leads to their gradual dissolution due to transformation into bicarbonate. As a result, huge cavities and caves appear in the earth's crust. Calcium bicarbonate transforms into calcium carbonate, forming stalactites and stalagmites (see figure).
Application of carbonic acid salts
One of the most widely used carbonic acid salts is sodium carbonate. It is known as soda ash and crystalline soda.
Soda ash is used in the production of soap, glass, for the production of inorganic dyes, in the production of aluminum, etc.
Acid salt - sodium bicarbonate is called baking soda. Baking soda is used in everyday life and in the food industry. If you add baking soda to the dough, then when baking products it decomposes, releasing carbon dioxide. This leads to loosening of the dough, and products made from it become more fluffy and porous.
Calcium carbonate, which exists in nature in the form of marble and limestone, is widely used in construction as facing and architectural building materials. In Figure 97 you see the Minsk metro station “Grushevka”, during the construction of which marble finishing was used.
Weak carbonic acid H2CO3 is formed when carbon dioxide is dissolved in water.
Carbonic acid forms two series of salts: acidic - hydrocarbonates and medium - carbonates.
Carbonates and hydrocarbonates are capable of mutual transformations.
Carbonates, as salts of a weak acid, react with all stronger acids to release carbon dioxide.
Salts of carbonic acid, except for the carbonates of most alkali metals, decompose when heated, releasing carbon dioxide.
The concept of organic substances
The total number of substances known today is enormous - there are more than 150 million of them! The vast majority of them are organic substances. They received this name because many of them were isolated from organisms .
One of the first such substances was probably fats. Early man, a hunter-gatherer, learned about them through the process of cooking. By roasting hunted animals over a fire or grinding the seeds of certain plants, he observed the release of viscous liquids that had similar properties. These substances were very nutritious and gave the body a lot of strength. People have long learned to extract fats from natural objects and have been using them as food or materials for obtaining other useful substances for many centuries. Today, everyone is familiar with fats of animal origin - pork fat, butter, as well as fats extracted from plants - sunflower, olive, flaxseed, palm, peanut and other oils.
While cooking meat over a fire, an ancient man accidentally made an important discovery. It turned out that drops of fat, falling on wet ash and cooling, gradually turned into a dense mass that foamed in the water and washed the dirt off the hands well. This is probably how people first became acquainted with soap, without which it is impossible to imagine our lives. Of course, today soap is made in a different way, but its basis is still fats.
Another important observation was made in ancient times. When crushed stems of one type of cane were squeezed, a liquid with a pleasant sweet taste was released. When this liquid was evaporated, a solid, even sweeter substance was obtained, called sugar. And from the liquid squeezed from potato tubers, a white substance called starch was isolated. Subsequently, it was found that sugar and starch are representatives of a large class of substances - carbohydrates.
By grinding grains of various cereals, people obtained flour, which, when mixed with water, forms dough for baking bread. In the first half of the 18th century. Gluten, an elastic and elastic mass, was isolated from dough for the first time. Subsequently, it turned out that it is a mixture of special substances - proteins, which can be of plant (gluten) and animal (chicken egg white) origin.
The vast majority of organic compounds currently known are non-natural substances - they are produced artificially in chemical laboratories or chemical plants (Fig. 98, 99). They are part of various valuable materials - synthetic fibers and rubbers, plastics and medicines, detergents and dyes, pesticides and fertilizers, explosives. Every week, thanks to scientific research, the number of organic substances increases by approximately 10,000.
All organic substances have a number of common properties that are unlike the properties of inorganic substances. How do organic substances differ from inorganic substances?
Firstly, in an amount that is more than 149 times greater than the number of inorganic compounds. Organic substances are incredibly diverse, and the number of classes of these compounds is tens of times greater than that of inorganic substances. The large number of organic substances and the diversity of their classes are due to the peculiarities of their composition and structure, which you will become familiar with in the next paragraph.
Secondly, the molecules of all organic substances necessarily include carbon atoms associated with atoms of a small number of elements - most often hydrogen, oxygen, nitrogen, sulfur, halogens, phosphorus. In this way, organic substances differ sharply from inorganic substances, which may contain atoms of all known chemical elements. Note that such simple carbon compounds as its oxides CO and carbonic acid and its salts are traditionally classified as inorganic substances.
Thirdly, many organic compounds are thermally unstable and, even at relatively low temperatures, decompose with the formation of carbon, that is, they become charred. When burned in oxygen, they form carbon dioxide and water. As for inorganic substances, most of them are thermally stable or decompose at very high temperatures. The products of their combustion in oxygen are a wide variety of substances.
Fourthly, organic substances are characterized by covalent polar and covalent nonpolar bonds. This also distinguishes organic substances from inorganic substances, which, in addition to the indicated types of bonds, also have ionic and metallic bonds.
Fifthly, almost all organic substances are compounds of molecular structure with low melting points. They are characterized by molecular crystal lattices. At the same time, most inorganic substances are compounds of non-molecular structure with high melting points. They are more characterized by atomic or ionic crystal lattices.
Despite the significant differences between organic and inorganic substances, their division into two groups is conditional. Both substances are formed and transformed in accordance with the same laws of nature. Organic and inorganic substances are united by their ability to transform each other. For example, as a result of photosynthesis, the organic substance glucose is formed from inorganic substances - carbon dioxide and water. Being a component of food, in human and animal organisms it again turns into its original inorganic compounds. This interconversion is the basis of the carbon cycle in nature.
Carbon is the basis of organic compounds
You have more than once observed clouds of thick black smoke escaping from the exhaust pipe of a faulty car running on diesel fuel (Fig. 100). It slowly rises up and mixes with the air, polluting it. Where does this smoke come from? Why is it black, although the liquid diesel fuel that the car is fueled with is transparent and almost
colorless? Here's the thing. The composition of this fuel includes various compounds of carbon with hydrogen, the so-called hydrocarbons, for example. When a working engine is running, they are mixed with air and completely burn, releasing heat, forming carbon dioxide and water, for example:
If the engine is faulty, then some of the hydrocarbons do not burn completely: oxygen from the air binds only with H atoms, and the remaining carbon atoms form a simple substance carbon, for example:
Carbon in the form of tiny black particles is forcefully ejected from the engine exhaust gases to the outside, forming a cloud of black smoke.
The formation of carbon from organic substances can also be observed in the school laboratory. Let's conduct an experiment. Pour some white glucose powder into a test tube and heat it in the flame of an alcohol lamp. First, the glucose will melt and turn into a viscous liquid, which, with further heating, will begin to foam and darken. After some time, droplets of water will form on the walls of the test tube, and a black solid substance - carbon - will remain at the bottom (Fig. 101).
Carbon is also formed by strong heating of other organic substances and materials based on them. What do the emission of black smoke from car exhaust pipes and the blackening of glucose when heated indicate? Of course, about the fact that the composition of molecules of organic substances
includes carbon atoms. Proof of this is the fact that with the complete combustion of organic substances in oxygen, carbon dioxide is always formed along with other substances
Carbon atoms bonded to atoms of other elements are present in the molecules of all organic substances without exception. For this reason, the branch of chemistry that studies these substances—organic chemistry—is called the chemistry of carbon compounds.
Why, out of 118 chemical elements, is carbon the basis of all organic substances? The answer to this question lies in the structural features of the atom of a given element.
Since carbon is a chemical element with atomic number 6, located in the second period of the periodic table, its atom has 6 electrons distributed over two electron layers. Since carbon is a group IVA element, the outer electron layer of its atom contains 4 electrons:
The electronic structure of an atom determines its following features.
1. Due to the presence of 4 electrons on the outer electron layer of the carbon atom, it does not have a pronounced ability to give or accept electrons and thus turn into ions. Therefore, carbon atoms form not ionic, but only covalent bonds, characteristic of molecules of organic substances.
2. Due to the fact that the radius of the carbon atom is small, the covalent bonds it forms are very strong. Carbon atoms form such covalent bonds with the atoms of most known chemical elements.
3. Since there are 4 electrons on the outer electron layer of the carbon atom, it exhibits a valency equal to IV - it forms four covalent bonds with other atoms. These can be four single bonds; two single and one double; two doubles; one single and one triple bond:
Here are examples of molecules of organic substances with such bonds:
4. Carbon atoms, unlike atoms of other elements, are capable of joining into chains of any length. They can be unbranched, branched and closed in cycles (Fig. 102):
And these are examples of molecules of organic substances with such chains of carbon atoms (Fig. 103):
Thus, atoms of only one chemical element - carbon - can combine both with atoms of other elements and with each other, forming chains or cycles in which carbon atoms are connected to other atoms by single, double or triple bonds. This uniqueness of carbon atoms is the basis for an incredibly large number of organic compounds and the diversity of their classes.
- Carbon atoms form covalent bonds, characteristic of molecules of organic substances.
- Carbon atoms form chemical bonds with atoms of most known elements.
- Carbon atoms are connected to other atoms by four covalent bonds (single or multiple).
- Carbon atoms are capable of connecting with each other into chains of any length, which can be unbranched, branched or closed in cycles.
The importance of organic substances in nature and human life
The world of organic substances is huge and diverse. Currently, according to their origin, they are divided into two groups. The first group includes organic compounds of natural origin that are part of all living organisms - humans, animals, plants, etc. They are also found in inanimate nature in the form of oil and natural gas. The second group consists of organic substances of non-natural (artificial) origin. Let's get acquainted with the role of organic substances in nature and human life.
Organic substances of natural origin
Organic substances of natural origin participate in all processes occurring in living organisms. The most important of them are proteins, fats, carbohydrates, vitamins, various acids, enzymes and hormones.
Proteins are vital substances whose molecules are chains of many thousands of atoms of carbon, hydrogen, oxygen, nitrogen and sulfur. In addition to the chicken egg protein that we have known since childhood, several million other proteins are known. They are found in the bodies of all living organisms and perform many functions. For example, proteins are part of muscles, bones, blood, form cartilage, skin, hair, nails, horns, hooves, feathers, scales (Fig. 104). Proteins are involved in muscle contraction processes and protect the body from infections. In living organisms, some proteins play the role of enzymes and hormones that regulate all vital processes.
In plant organisms, proteins are found in greatest quantities in seeds, where they are stored. The seeds of peas, beans, soybeans, and wheat grains are especially rich in protein.
Proteins are an important source of energy for humans and animals; they are part of food.
Some natural poisons are protein in nature and have a toxic effect on humans. These are proteins from the venom of snakes, some spiders, bees, wasps, as well as proteins from poisonous mushrooms, such as toadstools and fly agarics.
Along with proteins, nucleic acids . Their molecules, consisting of a huge number of carbon, hydrogen, oxygen, nitrogen and phosphorus atoms, are “templates” by which organisms synthesize the necessary proteins. Nucleic acids are a kind of memory devices with the help of which each type of living organism passes on the “recording” of the structure of its proteins from generation to generation.
Fats are complex organic substances containing carbon, hydrogen and oxygen atoms. They are found in human bodies, animals, plants, etc. Everyone knows fats of animal origin, for example pork, beef, lamb fat, butter (Fig. 105). Vegetable fats are called oils. These include sunflower, flaxseed, rapeseed, olive, peanut, palm and other oils. They accumulate in seeds or fruits of plants. You can check this by placing a sunflower seed on a piece of paper and pressing it firmly. An oily stain will appear on the paper.
Fats are the most important source of energy for humans and an integral part of food. By forming fat capsules, fats protect internal organs from shock and protect the body from hypothermia. The fats secreted by the sebaceous glands of the skin make human skin soft and elastic, and hair shiny. Together with proteins, fats are a reserve building material from which new body cells are formed.
Carbohydrates are complex organic substances whose molecules consist of carbon, hydrogen and oxygen atoms. Carbohydrates are formed in green plants during photosynthesis from carbon dioxide and water. They are part of the cells and tissues of all plant and animal organisms and, by mass, make up the bulk of organic matter on Earth.
Green plants annually absorb approximately 200 billion tons of carbon dioxide CO3 from the atmosphere through the process of photosynthesis. At the same time, about 130 billion tons of oxygen O2 enters the atmosphere and 50 billion tons of carbohydrates are synthesized.
Animal organisms are not able to synthesize carbohydrates, so they obtain them from plant sources. Carbohydrates include, for example, glucose, fructose, sucrose, starch, cellulose, etc. Glucose, fructose and sucrose are found in the juice of vegetables and fruits, giving them a sweet taste. Glucose is an essential component of the human body. Sugar beets and sugar cane are rich in sucrose - the main sources of sugar. Starch accumulates in tubers, fruits, and plant seeds. Thus, potato tubers contain up to 24% starch, wheat grains - up to 64%, rice - 75%, corn - 70%. Glucose, fructose, sucrose and starch are important sources of energy for humans. They are easily digestible and are included in food products. Cellulose (fiber) is a carbohydrate that makes up the cell walls of all higher plants. Cellulose is familiar to every person and is found literally at every step. Poplar fluff and dandelion parachutes, cotton wool made from cotton seeds (Fig. 106), flax, straw, paper - all this is almost pure cellulose. It is part of such an important material as wood. In mammals, which includes humans, cellulose is not digestible. However, it is the main food of many herbivores, such as cows, sheep, horses, and deer.
Vitamins are organic substances that do not supply energy to the body, but are needed in small quantities to maintain life. Natural sources of vitamins are vitamin-rich plants (rose hips, citrus fruits, parsley, onions, cabbage, carrots, currants, rowan, sea buckthorn, etc.), as well as some food products of animal origin. Many vitamins today are obtained synthetically.
Vitamins enter the body with food and are involved in almost all processes occurring in our body. They are necessary for the normal functioning of the endocrine glands, increasing mental and physical performance, and the body’s resistance to the effects of adverse environmental factors (heat, cold, infections, poisoning). Currently, about 20 different vitamins are known. This is, for example, vitamin C - the familiar “ascorbic acid”, as well as vitamins, etc. A lack of vitamins, as well as their excess in the body, cause various diseases.
Place in the periodic table
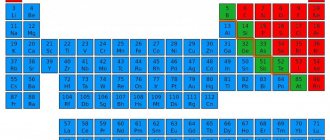
The arrangement of chemical elements in the periodic table of Mendeleev is based on their atomic weight, calculated relative to the atomic weight of hydrogen. The atomic mass of carbon is 12.011, according to which it occupies an honorable 6th place in the periodic table and is designated by the Latin letter C.
In addition, you should pay attention to the following characteristics of carbon:
Graphite
Most often in nature, pure carbon can be found in the form of graphite - a soft black material that exfoliates easily and seems slippery to the touch. Many may remember that pencil leads are made from graphite - but this is not always true. Often the lead is made from a composite of graphite chips and glue, but there are also completely graphite pencils. Interestingly, more than one twentieth of the world's natural graphite production goes into pencils.
What is special about graphite? First of all, it conducts electricity well - although carbon itself is not like other metals. If you take a graphite plate, it turns out that along its plane the conductivity is about a hundred times greater than in the transverse direction. This is directly related to how the carbon atoms in the material are organized.
If we look at the structure of graphite, we will see that it consists of individual layers one atom thick. Each layer is a grid of hexagons, reminiscent of a honeycomb. The carbon atoms inside the layer are linked by covalent chemical bonds. Moreover, some of the electrons that provide chemical bonding are “smeared” over the entire plane. The ease of their movement determines the high conductivity of graphite along the plane of carbon flakes.
The individual layers are connected to each other thanks to van der Waals forces - they are much weaker than a conventional chemical bond, but are sufficient to ensure that the graphite crystal does not delaminate spontaneously. This discrepancy makes it much more difficult for electrons to move perpendicular to the planes - electrical resistance increases 100 times.
Due to its electrical conductivity, as well as the ability to embed atoms of other elements between layers, graphite is used as anodes for lithium-ion batteries and other current sources. Graphite electrodes are necessary for the production of aluminum metal - and even trolleybuses use graphite sliding contacts for current collectors.
In addition, graphite is a diamagnetic material, and has one of the highest susceptibility per unit mass. This means that if you place a piece of graphite in a magnetic field, it will try in every possible way to push this field out of itself - to the point that the graphite can levitate above a sufficiently strong magnet.
And the last important property of graphite is its incredible refractoriness. The most refractory substance today is considered to be one of the hafnium carbides with a melting point of about 4000 degrees Celsius. However, if you try to melt graphite, then at pressures of about one hundred atmospheres it will retain hardness up to 4800 degrees Celsius (at atmospheric pressure, graphite sublimates - evaporates, bypassing the liquid phase). Due to this, graphite-based materials are used, for example, in rocket nozzle housings.
Physical properties
With its physical properties, carbon is a typical non-metal. At the same time, it forms many allotropic modifications (“allotropic” means the existence of two or more different substances from one chemical element): the most popular of them are diamond, graphite, coal, soot. Moreover, diamond is one of the hardest carbon substances.
Of course, different allotropic modifications of carbon also have different physical properties. If diamond is a typical solid, then, for example, liquid carbon, which can only be obtained under a certain external pressure, has completely different physical properties than diamond or graphite.
Graphene
Instead of compressing and heating graphite, we, following Andrei Geim and Konstantin Novoselov, will glue a piece of tape to the graphite crystal. Then peel it off - a thin layer of graphite will remain on the tape. Let's repeat this operation again - apply the tape to a thin layer and peel it off again. The layer will become even thinner. By repeating the procedure a few more times, we obtain graphene, the material for which the aforementioned British physicists received the Nobel Prize in 2010.
Graphene is a flat monolayer of carbon atoms, completely identical to the atomic layers of graphite. Its popularity is due to the unusual behavior of the electrons in it. They move as if they have no mass at all. In reality, of course, the mass of electrons remains the same as in any substance. The carbon atoms of the graphene frame are to blame for everything, attracting charged particles and forming a special periodic field.
Graphene-based device. In the background of the photo are gold contacts, above them is graphene, above is a thin layer of polymethyl methacrylate
Engineering at Cambridge / flickr.com
Share
The consequence of this behavior is the greater mobility of electrons - they move in graphene much faster than in silicon. For this reason, many scientists hope that graphene will become the basis of the electronics of the future.
Interestingly, graphene has carbon cousins - pentagraphene and phagraphene. The first of them consists of slightly distorted pentagonal sections and, unlike graphene, does not conduct electricity well. Phagraphene consists of pentagonal, hexagonal and heptagonal sections. If the properties of graphene are the same in all directions, then phagraphene will have a pronounced anisotropy of properties. Both of these materials have been theoretically predicted, but do not yet exist in reality.
A fragment of a silicon single crystal (in the foreground) on a vertical array of carbon nanotubes
zeiss.com
Chemical properties
Under normal conditions, carbon is generally chemically inert, but at high temperatures it can react chemically with many other elements, usually exhibiting strong reducing properties. Here are examples of chemical reactions of carbon as a reducing agent with:
— with oxygen C0 + O2 –t°= CO2 carbon dioxide
with a lack of oxygen - incomplete combustion: 2C0 + O2 –t°= 2C+2O carbon monoxide
— with fluorine C + 2F2 = CF4
— with water vapor C0 + H2O –1200°= C+2O + H2 water gas
- with metal oxides. In this way, metal is smelted from ore. C0 + 2CuO –t°= 2Cu + C+4O2
- with acids - oxidizing agents: C0 + 2H2SO4 (conc.) = C + 4O2 + 2SO2 + 2H2O C0 + 4HNO3 (conc.) = C + 4O2 + 4NO2 + 2H2O
— with sulfur forms carbon disulfide: C + 2S2 = CS2.
Sometimes carbon can also act as an oxidizing agent, forming carbides when entering into chemical reactions with certain metals:
4Al + 3C0 = Al4C3
Ca + 2C0 = CaC2-4
When carbon reacts with hydrogen, it forms methane:
C0 + 2H2 = CH4
Fullerenes
Although the hexagon is one of the most stable configurations that carbon atoms can form, there is a whole class of compact objects where the regular carbon pentagon occurs. These objects are called fullerenes.
In 1985, Harold Kroteau, Robert Curl, and Richard Smalley studied carbon vapor and how carbon atoms clump together when cooled. It turned out that there are two classes of objects in the gas phase. The first is clusters consisting of 2–25 atoms: chains, rings and other simple structures. The second is clusters consisting of 40–150 atoms, which have not been observed before. Over the next five years, chemists were able to prove that this second class consists of hollow frameworks of carbon atoms, the most stable of which consists of 60 atoms and is shaped like a soccer ball. C60, or buckminsterfullerene, consisted of twenty hexagonal sections and 12 pentagonal sections, bonded together to form a sphere.
The discovery of fullerenes aroused great interest among chemists. Subsequently, an unusual class of endofullerenes was synthesized - fullerenes in the cavity of which there was some foreign atom or small molecule. For example, just a year ago a hydrofluoric acid molecule was placed into a fullerene for the first time, which made it possible to very accurately determine its electronic properties.
Fullerites - crystals of fullerenes
Wikimedia Commons
Share
In 1991, it turned out that fullerides—fullerene crystals in which some of the cavities between adjacent polyhedra are occupied by metals—are molecular superconductors with a record high transition temperature for this class, namely 18 kelvin (for K3C60). Later, fullerides with an even higher transition temperature were found - 33 kelvin, Cs2RbC60. Such properties turned out to be directly related to the electronic structure of the substance.
Role in nature
The earth's crust contains only 0.15% carbon. Despite this seemingly small figure, it is worth noting that carbon continuously participates in the natural cycle from the earth’s crust through the biosphere to the atmosphere and vice versa. It is also carbon that makes up such valuable resources as oil, coal, peat, limestone and natural gas. And as we wrote at the beginning of our article, carbon is the basis of life. Let's say that the body of an adult weighing 70 kg contains about 13 kg of carbon. This is only in one person; carbon is contained in approximately the same proportions in the bodies of all other living beings, plants and animals.
Application
It can be said that carbon is inextricably linked with the very development of human civilization. It is from compounds containing carbon that the main fuels are formed, thanks to which cars drive, planes fly, you can cook your own food and heat your home in the cold season - these are oil and gas. In addition, carbon compounds are actively used in the chemical and metallurgical industries, pharmaceuticals and construction. Diamonds, being an allotropic modification of carbon, are used in jewelry and rocket science. In general, modern industry cannot do without carbon; it is needed almost everywhere.
Carbin
When talking about elongated structures of carbon atoms, one cannot fail to mention carbines. These are linear chains, which, according to theorists, may turn out to be the strongest material possible (we are talking about specific strength). For example, the Young's modulus for carbyne is estimated at 10 giganewtons per kilogram. For steel this figure is 400 times less, for graphene it is at least two times less.
A thin thread stretching to the iron particle below - carbine
Wikimedia Commons
Share
Carbynes come in two types, depending on how the bonds between the carbon atoms are arranged. If all the bonds in the chain are the same, then we are talking about cumulenes, but if the bonds alternate (single-triple-single-triple and so on), then we are talking about polyines. Physicists have shown that a carbyne thread can be “switched” between these two types by deformation - when stretched, cumulene turns into polyyne. Interestingly, this radically changes the electrical properties of carbyne. While polyyne conducts electric current, cumulene is a dielectric.
The main difficulty in studying carbines is that they are very difficult to synthesize. These are chemically active substances that are also easily oxidized. To date, chains of only six thousand atoms in length have been obtained. To achieve this, chemists had to grow carbyne inside a carbon nanotube. In addition, the synthesis of carbyne will help break the record for the size of the gate in a transistor - it can be reduced to one atom.
Recommended reading and useful links
- Savvatimskiy, A (2005). “Measurements of the melting point of graphite and the properties of liquid carbon (a review for 1963–2003).” Carbon. 43(6):1115–1142. doi:10.1016/j.carbon.2004.12.027
- Chemical Encyclopedia / Editorial Board: Knunyants I.L. and others. - M.: Soviet Encyclopedia, 1988. - T. 1. - 623 p.
- ChemNet. Carbon: the history of the discovery of the element.
- Leypunsky O.I. About artificial diamonds (Russian) // Advances in chemistry. - Russian Academy of Sciences, 1939. - Issue. 8. - pp. 1519-1534.
- Seal M. The effect of surface orientation on the graphitization of diamond. //Phis. Stat. Sol., 1963, v. 3, p. 658.
Video
And finally, an educational video on the topic of our article.
Author: Pavel Chaika, editor-in-chief of Poznavaika magazine
When writing the article, I tried to make it as interesting, useful and high-quality as possible. I would be grateful for any feedback and constructive criticism in the form of comments on the article. You can also write your wish/question/suggestion to my email [email protected] or Facebook, with respect, the author.
Author page