Author: Kolesnikov Yuri Fedorovich, heating engineer*
© When using site materials (quotes, images), the source must be indicated.
Crucible is a vessel for melting metal. As a rule, conversion metal is melted in crucibles, i.e. already brought to the required degree of quality for casting into a mold or refining (deep purification from impurities). The general line of development of large-scale metallurgy is to reduce the number of processing steps, up to the release of conditioned metal directly from the melting furnace, but in industry crucible melting still retains significant importance, and in handicrafts and jewelry it dominates.
The crucible is not just a fairly heat-resistant vessel. Its chemical composition and design must correspond to the type of metal being melted and the melting mode. This article describes how to make a crucible with your own hands and what conditions it must satisfy for use at home or in a small workshop. For beginner metallurgists, you will first have to touch on the metal smelting process itself, because... The requirements for the crucible are determined mainly by its conditions.
Melting metal in a crucible at home
A little about melting
In a deep vacuum, the high-purity metal being melted can be heated exactly to the melting temperature or slightly higher, and kept at it for some time so that tiny, literally a few atoms, remains of crystallites melt. Then the metal can be allowed to cool slightly below its melting point - it will remain liquid, like a supersaturated solution without a seed crystal. If we now pour the metal, also in a vacuum, into a mold made of a chemically absolutely inert material, in which a seed crystal of the same metal is placed, then, observing all the subtleties of this technology, we will obtain a single-crystal casting with unique properties.
In amateur conditions, vacuum melting, alas, is not feasible. In order to properly make a crucible for melting metal yourself, you need to take into account a number of features of melting in a non-inert chemical gas environment. The melted metal, firstly, interacts with air, causing part of it to be lost to the formation of oxide, which is especially important when melting scrap precious metals: at its melting temperature (1060 degrees Celsius), even gold noticeably oxidizes. To compensate to some extent for oxidation, the crucible must provide a reducing environment for the melt or be chemically inert if the metal is melted with a clean open flame, see below.
Secondly, so that the metal in the crucible does not freeze until it is brought to the casting mold, so that the remnants of the original crystallites do not spoil the casting, and the melt acquires sufficient fluidity, the metal in the crucible is overheated. For example, the melting point of zinc is 440 degrees, and its foundry temperature is 600. Aluminum, respectively, 660 and 800. Since overheating of the metal after melting takes some time, degassing of the melt also occurs at the same time, this is the third thing.
Recovery
In metallurgy, atomic carbon C, carbon monoxide CO (carbon monoxide) and hydrogen H are used as reducing agents. The latter is most often an accidental guest, because for this purpose it is too active and is absorbed by metals without forming chemical compounds with them in large quantities, which spoils the casting material. For example, solid platinum at room temperature can absorb up to 800 volumes of hydrogen. A platinum blank in a hydrogen atmosphere literally swells before our eyes, cracks and falls into pieces. If you take them out of the hydrogen chamber and heat them, hydrogen will be released back.
Note: in a similar way, but in smaller quantities, metals absorb/emit other gases, e.g. nitrogen. This is why degassing of the melt is required, see also below.
A noticeable proportion of hydrogen reduction occurs when heated by an open flame of a gas burner, upon its contact with a less heated surface. The metal does not deteriorate - the absorbed hydrogen is released and burned later in the smelting process. But, if the crucible material is also prone to gas absorption, it may crack and burst during melting; this must be kept in mind.
CO reduction is noticeable if the metal in the crucible is melted by the open flame of a liquid (gasoline, kerosene, diesel) burner, for the same reasons. Liquid fuel burns much slower than gas, and its afterburning zone extends several cm from the burner nozzle. Reduction with carbon monoxide is the cleanest from the point of view of the metal: it does not spoil the metal and does not produce by-products with a strong excess of the reducing agent. Therefore, CO reduction is widely used in metallurgy when smelting metal from ore, but no one has yet figured out how to make a crucible furnace (see below), in which oxidation compensation would be completely provided by CO.
Atomic carbon is a reducing agent energetic enough to compensate for oxidation. It is also not difficult to create a reducing environment in a crucible using C: it is enough to introduce free carbon in one or another allotropic modification into the composition of its material or make the entire crucible from a heat-resistant and mechanically sufficiently strong allotrope C; graphite is one of them. When reducing C, there is a danger of carburization of the melt, but graphite releases very little atomic carbon when heated. If you heat the metal in a graphite crucible with a gas flame, then the excess C will immediately find a more “tasty” H for it and the danger of carburization will be reduced to zero. And for other heating methods (see below), you can select the dimensions, configuration of the crucible and the addition of graphite to its material so that there is simply no excess C under any conceivable melting mode. This is a very valuable property of graphite, keep it in mind too.
Note: the coefficient of thermal expansion of TKR graphite is negative, which significantly compensates for the thermal expansion of the crucible, increases its durability and increases its service life. Also valuable quality.
Excerpt
So, it’s clear why the melt in the crucible needs to be overheated and held. Although metal casting is a completely different topic, it still needs to be mentioned here that the melt holding time should be observed quite accurately. Chemically pure metals are almost never used in practice, for example. gold 9999 wears out very quickly; The exception is electrical copper and zinc for galvanizing, the cleaner they are, the better. Most often they use the so-called. eutectic alloys; eg steel is a eutectic of iron and carbon, and duralumin is a complex eutectic of several components. If the melt is allowed to sit, the structure of the eutectic in the casting will change and the finished product will be spoiled. The holding time is especially critical for bronze and brass: they need to be cast immediately, as soon as the play of the melt in the crucible apparently changes and becomes calmer. Remember how the engineer Telegin in A. N. Tolstoy’s “Walking Through Torment” was worried that the bronze would not wear out?
Read also: DIY mini snowmobiles video free
In relation to the manufacture of a homemade crucible, degassing of the melt during exposure is significant in that at this time it (the crucible) experiences significant dynamic loads from bubbles of released gases and/or the play of the melt itself. That is, make the crucible withstand a large amount of thermal deformation and, if recovery is required, a small amount. Its material must also be viscous enough to withstand shock waves from bursting bubbles and shocks from melt jets. It is this circumstance that explains the low durability and reliability of homemade graphite crucibles (see below).
What to make from
Melting crucibles are made (see figure below):
- ceramic chemically neutral;
- ceramic graphite;
- graphite;
- cast iron;
- steel.
Crucibles for melting metal from various materials
Their comparative characteristics are as follows:
- Ceramic neutral - used for melting scrap jewelry while preserving the sample, because with indirect heating (see below), the properties of the metal do not change. You can do it yourself, but it’s a little complicated (see below) and is it worth it? A 50 g gold crucible costs up to 100 rubles in a jewelry store. Without any problems, they are suitable for melting in an induction furnace (see below), because almost do not absorb the energy of the electromagnetic field (EMF). Resource – 10-30 melts.
- Ceramic graphite – suitable for melting any metal; at home up to 1.5-2 kg at a time. To use an induction furnace, its power for the same amount of metal will have to be increased by 1.5-2 times due to the absorption of EMF by conductive graphite. You can do it yourself, see below. Resource – up to 50 or more melts.
- Graphite - suitable for melting old, oxidized scrap non-ferrous and precious metals, because create a strong restorative environment. Melting silver with an open gas flame in a graphite crucible makes it possible to almost completely restore the original weight of the oxidized metal. You can’t do it yourself, see below. Resource – more than 100 melts.
- Cast iron - used mainly for melting red copper into oxygen-free copper, because actively absorb oxygen. The resource is up to 30 melts, and then the amorphous carbon leaves the cast iron and the crucible degrades.
- Steel - a homemade cheap option for melting small quantities of aluminum and magnesium alloys and other chemically inert metals in the melt. Can be used for melting small amounts of lead into fishing weights, etc.
Note: graphite, cast iron and steel crucibles for use in induction furnaces (see below) are completely unsuitable, because completely absorb EMF energy.
About graphite crucibles
Graphite crucibles are made either turned from massive natural graphite (expensive), or sintered at high temperatures from graphite powder (cheaper, but still not very cheap). Hobbyists often try to make “graphite” crucibles from ground graphite with a kaolin binder, etc., but what they end up with is not graphite, but overly graphitized ceramic crucibles - fragile, withstanding no more than 10 melts and spoiling the metal due to excessive release of atomic carbon by finely dispersed graphite . A more or less rational way to use ground graphite in amateur crucible melting is to make a tabletop mini crucible furnace from it for ceramic neutral crucibles, see fig.
Graphite Mini Furnace for Jewelry Crucible Heating
Cold welding for assembling this furnace should be used at a temperature of at least 800 degrees - the cheeks, which conduct electricity well, do not heat up above 400 during one melt. Graphite powder will not heat up much more without a crucible, but when the crucible is pressed into it, it will be hot spot over 1000 degrees due to compaction of the powder under the crucible.
If gold is melting, then after the melting is completed and the furnace has cooled, the graphite powder is poured out and shaken, because it gets baked. To melt silver and cupronickel, the powder is removed and shaken after 3-5 melts, so the furnace heats up faster. In any case, to maintain a reducing environment, the furnace is covered with a mica lid during melting.
Graphite crucible
A crucible is a fireproof container designed for melting metals. In crucibles containing graphite, steel or copper and alloys based on it are melted. The production of molten metal is carried out in crucible furnaces.
This melting method is limited by the volume of melt produced. Graphite crucibles are used in laboratories, factories to produce rare alloys or to produce castings from copper and precious metals, as well as in home workshops.
Heating methods
If you need to melt more than 150-200 g of metal at a time, then you will need to build a crucible furnace next to the crucible, otherwise it will be very difficult to achieve homogeneity of the melt and high quality casting. The exception is low-melting and easily recoverable lead: up to 20-30 kg of it can be melted at a time at home. A relative exception is zinc for hot galvanizing; its melt in a crucible without a furnace can be up to 2-2.5 kg, but borax must be sprinkled on top of it so that the surface of the melt is completely covered with its fluidized layer. Steel fasteners are thrown into the melt through a layer of borax.
The optimal method in all respects for heating the crucible in a furnace is with gas, pos. 1 in Fig., but a gas crucible furnace is a rather complex structure, although it can easily be made independently. The most suitable crucible for a gas furnace is a graphite ceramic crucible, because its material has fairly high thermal conductivity. If there are particularly high requirements for metal purity, it is better to use a neutral ceramic crucible. When lower for fusible metals - cast iron, as it conducts heat better and thereby saves fuel. Graphite crucibles are placed in a gas furnace only if strong reduction of old oxidized metal is required, and the danger of carburization is insignificant, for example, when melting silver extracted from the earth for refining
Methods for melting metal in a crucible
For low-melting metals, the electric crucible furnace, pos. 2; it may be the so-called ohmic (with heating by a nichrome spiral) or induction, with heating from an electromagnetic oscillation generator, see below. Only ceramic neutral or, to a limited extent, graphite crucibles are suitable for induction furnaces.
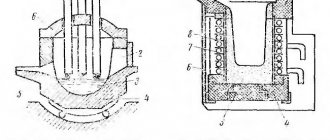
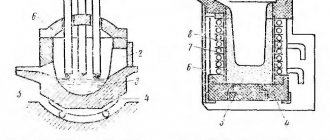
If the crucible contains more than 2-2.5 kg of metal, then according to safety rules the crucible furnace must be made tiltable (item 3), because and 1 kg of melt spilled on the floor is already a big disaster. On the contrary, it is preferable to heat metal in small jewelry crucibles without a furnace, directly with the flame of a burner, pos. 4. In this case, the crucible is held throughout the melting process with a special spring grip, pos. 5 and 6.
Note: silver and its alloys, as well as lead on sinkers, can be melted at home in quantities of up to 15-20 g, using instead of a crucible... a food-grade stainless steel spoon, see fig. on right. For safety, then it is necessary to make gaskets for the jaws of the vice with longitudinal cuts under the handle of the spoon. The flame is exclusively gas; gasoline can burn a spoon.
Read also: Molds for casting lead weights
Electric heating
Ohmic crucible furnaces are mainly used for smelting lead or tin. For more refractory metals, they turn out to be uneconomical, but up to 20 kg of lead can be melted at a time in a home crucible electric furnace; how to make your own electric crucible for melting lead, see for example. video:
Video: electric crucible for melting lead
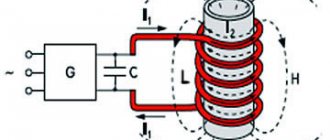
Melting aluminum in a crucible turns out to be more profitable by induction due to its high electrical conductivity, but this trick no longer works with copper - its temperature and latent heat of fusion are much higher. With the induction melting method, the metal is heated by Foucault eddy currents, for which the crucible with it is placed in an EMF coil of thick copper wire, powered by alternating current from an electromagnetic oscillation generator. How to make a generator with your own hands for inductively heating small amounts of metal, for example, for trinkets, is described in other materials, or, for example, see next. video guide.
Video: DIY induction heating
Induction crucible furnace for aluminum melting
With an increase in the amount of melted metal, not only does the required power of the generator increase, but its optimal frequency also decreases, this affects the so-called. surface effect (skin effect) in metal. If 100-200 g of aluminum can be melted into EMF from any homemade generator for inductive heating, then installation of 1.5-2 kg of duralumin or magnesium alloy is already a solid structure, see fig. on right. If you intend to work with aluminum, then think carefully - is it worth building something like this? Wouldn't it be easier to use a mini gas furnace for melting small quantities of aluminum alloys, see for example. video clip
Video: mini furnace for melting aluminum
How to make a crucible or melting furnace with your own hands
Almost every item has several types and purposes, including stoves. There are stoves for heating rooms and for cooking food, and there are special devices for melting metals or storing them in molten form. Such devices are called crucible melting furnaces. They have a specific purpose and therefore the list of enterprises where they have found their application is quite small. These are mainly factories and laboratories. But what to do if you need to melt metal for some purpose at home? Buying such equipment is very expensive, but it is quite possible to make it yourself. This requires minimal knowledge in this area, desire and time.
Making crucibles
Now it's time to make your own melting crucible. From the above it is clear that it makes sense to make crucibles with your own hands:
- Steel;
- Ceramic neutral;
- Ceramic graphite.
There is nothing special to say about steel crucibles - they are just a steel vessel with a welded handle. Steel crucibles are used for melting low-melting metals; sometimes - zinc for hot galvanizing with quality up to 3+. Steel crucibles for lead, tin and zinc are only suitable for melting one specific metal, because... after 1-2 melts they themselves are covered with it from the inside.
Other types of crucibles
Crucibles are made not only from graphite, but also from cast iron and ceramics. Cast iron containers are used quite rarely due to: rapid oxidation of iron, its entry into a chemical reaction with the melt, high reactivity, low heat resistance and fire resistance. This is why cast iron models have a low cost.
Ceramic crucibles of various shapes
No reactions or changes occur in ceramic crucibles. Therefore, they are used for melting chrome, cobalt alloys and base metals.
Quartz crucible inserts
As an option, inserts into the crucible are made from quartz. They are used when it is necessary to exclude the interaction of the melt with graphite or cast iron.